Fully Uncomplexed Cyclodextrin in Mixed Systems of Vesicle-Cyclodextrin- Solvolysis of

Fully Uncomplexed Cyclodextrin in Mixed Systems of Vesicle -Cyclodextrin:Solvolysis of Benzoyl Chlorides
C.Cabaleiro-Lago,?L.García-R?′o,?P.Herve ′s,*,?and J.Pe ′rez-Juste ?
Department of Physical Chemistry,Faculty of Chemistry,Uni V ersity of Vigo,36310Vigo,Spain,and Department of Physical Chemistry,Faculty of Chemistry,Uni V ersity of Santiago de Compostela,15782Santiago de Compostela,Spain
Recei V ed:February 4,2009;Re V ised Manuscript Recei V ed:March 11,2009
In this contribution the in?uence of -cyclodextrin (CD)on the behavior of aqueous systems containing vesicles of dipalmitoyl phosphatidyl choline (DPPC)has been studied by determining the kinetics of the solvolysis reaction of substituted benzoyl chlorides whose solvolysis reactivity entails a high sensitivity on media properties.The application of the pseudophase formalism allowed us to obtain the thermodynamic and kinetic coef?cients characteristic of the reaction,which are essentially independent of the concentration of CD.We were able to determine the percentages of uncomplexed cyclodextrin in equilibrium with the vesicular system which were in all cases compatible with 100%.The obtained results led us to conclude that the properties of DPPC vesicles are not affected by the presence of CD in the medium and there is no type of interaction between the CD and the vesicular surfactant monomers and,therefore,all cyclodextrin is present in the mixed system as uncomplexed cyclodextrin.
Introduction
There is increasing interest in investigating surfactant ag-gregates that mimic biological membranes,such as phospho-lipidic,liposomes or synthetic amphiphile vesicles,because the architecture of these arti?cial membranes is considerably simpler than that of cell membranes.1,2Membrane mimetic agents have been used in reactivity control,photochemical reactions,and provided unique environments for substrates and enzymes.Liposomes are useful as both biomembrane models and potential drug carriers.3Since liposomes are closed vesicles consisting of unilamellar or multilamellar membranes,they can encapsulate various molecules in their internal aqueous phase or their phospholipid membranes.4In fact,liposomes carrying antibodies or oligosaccharide chains have also been reported as effective and speci?c reagents in antibacterial,antitumor,5and antihuman inmmunode?ciency virus (HIV)therapies.6Kinetically,vesicles make a highly appealing reaction medium.Because of their physical and chemical characteristics,they can inhibit chemical reactions 7-9or they can catalyze reactions acting as micro-reactors.7-15
Cyclodextrins (CD)also have the ability to alter chemical reactivity.The most studied cyclodextrins are R -, -,and γ-cyclodextrins,which consist in six,seven,and eight glucose units,respectively.16Regardless of the ?ner details of their structure,the most important feature of CDs is their cavity,because this enables them to form inclusion complexes with a great variety of substrates.16-18Increasingly,the native CDs now serve as scaffolds on which multiple functional groups can be assembled with controlled geometry opening new areas of supramolecular chemistry.19-22
Cyclodextrins as drug complexing agents have been the object of intense interest for both fundamental aspects and practical
purposes for a long time.22-24Recently,this attention has turned to the problem of biological photosensitization of drugs.25Indeed,despite their excellent therapeutic activity,many pharmacologically important chemicals such as antibacterials,antimicotics,and nonsteroidal anti-in?ammatory drugs can induce phototoxic,photoallergic,and photomutagenic phenom-ena strictly related to the drug photochemical reactivity.26It has been reported that in some cases such effects can be substantially decreased in the presence of CDs with model cellular systems.27-29Application of CDs was,therefore,suggested as a useful strategy to minimize the biological damage induced by drugs and increase drug photostability.However,it should be stressed that drug -CD complexes usually dissociate once introduced into the body,where there is also exposure to a wide range of endogenous species.29,30
In the work described in this article we studied the in?uence of -cyclodextrin (CD)on the behavior of aqueous systems containing vesicles of dipalmitoyl phosphatidyl choline (DPPC)by determining the kinetics,in these media,of the solvolysis reaction of substituted benzoyl chlorides (see Scheme 1).Benzoyl chlorides’geometry and polarity give rise to the formation of an inclusion complex with CD,31and their solvolysis reactivity entails a high sensitivity on media properties.32-34It is known that the addition of enough amounts of cyclodextrins to micellar systems causes their destruction due to the formation of CD -surfactant monomer complexes.Previ-
*To whom correspondence should be addressed.E-mail:jherves@uvigo.es.?
University of Vigo.?
University of Santiago de Compostela.
SCHEME
1
J.Phys.Chem.B 2009,113,6749–67556749
10.1021/jp901028k CCC:$40.75 2009American Chemical Society
Published on Web
04/20/2009
ous studies carried out in our group have shown that,at the micellization point,appreciable concentrations of uncomplexed CD exist.35Furthermore,the increase of the hydrophobic character of the micellar surfactant monomers leads to an increase in the percentage of uncomplexed CD.36The aim of the present work was to study a mixed system composed by cyclodextrins and vesicles (more hydrophobic than micelles and also more realistic models for biological membranes),in which it is possible that the presence of cyclodextrins neither destroys nor alters the properties of the vesicles.Experimental Section
All the reagents (from Sigma)were of the highest available grade and used without further puri?cation.The stock solutions of benzoyl chlorides were prepared in acetonitrile to prevent them from decomposing too rapidly.CD solutions were made taking into account that commercial CD has a H 2O content of 8mol mol -1.For aqueous solutions double-distilled and deionized water was used.All experiments were carried out at 25.0(0.1°C.
Vesicle Preparation.DPPC stock solutions were prepared by weighing the required amount of solute,adding water,and keeping the solution for 30min in a water bath at 65°C.Then the solution was sonicated with a tip sonicator (Bandelin UW 2200)for 30min at 65°C (in some cases,DPPC solutions were cosonicated in presence of cyclodextrin).After those samples were equilibrated to room temperature and ?ltered through a 0.45μm pore size ?lter twice,the stock solution was diluted to the desired concentrations to prepare samples for kinetic measurements.Although the vesicles were assumed to be stable,we always used dispersions within 3h after preparation.
Dynamic Light Scattering Measurements.Samples were irradiated with an Ar +laser at λ)514.5nm,and data were recorded at three different angles (60°,90°,and 120°).Scattering data were analyzed by means of a Malvern autosizer 4700digital correlator.Correlation functions were ?tted by using the CONTIN and cumulants methods.
Transmision Electron Microscopy (TEM).Vesicles were imaged with a JEOL JEM-1010transmission electron micro-
scope using the negative-staining method.A drop of vesicle solution was spread on a 200mesh copper grid coated with a Formvar ?lm,and the extra droplet was instantly wiped off by ?lter paper.After being naturally desiccated,a drop of 2%uranyl acetate in ethanol solution was dripped on the copper grid for about 60s and the extra droplet was also removed.Then the grid was dried naturally for about 3h before TEM observation.Kinetic Measurements.Solvolysis reactions were carried out in an Applied Photophysics SX-18MV stopped-?ow reaction analyzer thermostatted with a Polyscience water bath.All kinetic experiments were performed using a 1:25asymmetric mixing kit so that the percentage of acetonitrile in the reaction mixture was always less than 4%by volume.Kinetic pro?les were followed by monitoring the decrease in absorbance of benzoyl chlorides.The wavelengths used for the kinetic studies ranged between 250and 300nm for 4-MeO,4-Cl,and 4-CF 3.The concentration range was between 6and 9×10-4M.Kinetic data were always satisfactorily ?tted by the ?rst-order integrated rate equations,and therefore,in what follows,k obs denotes the pseudo-?rst-order rate constant.Experiments were reproducible to within 5%.
Results
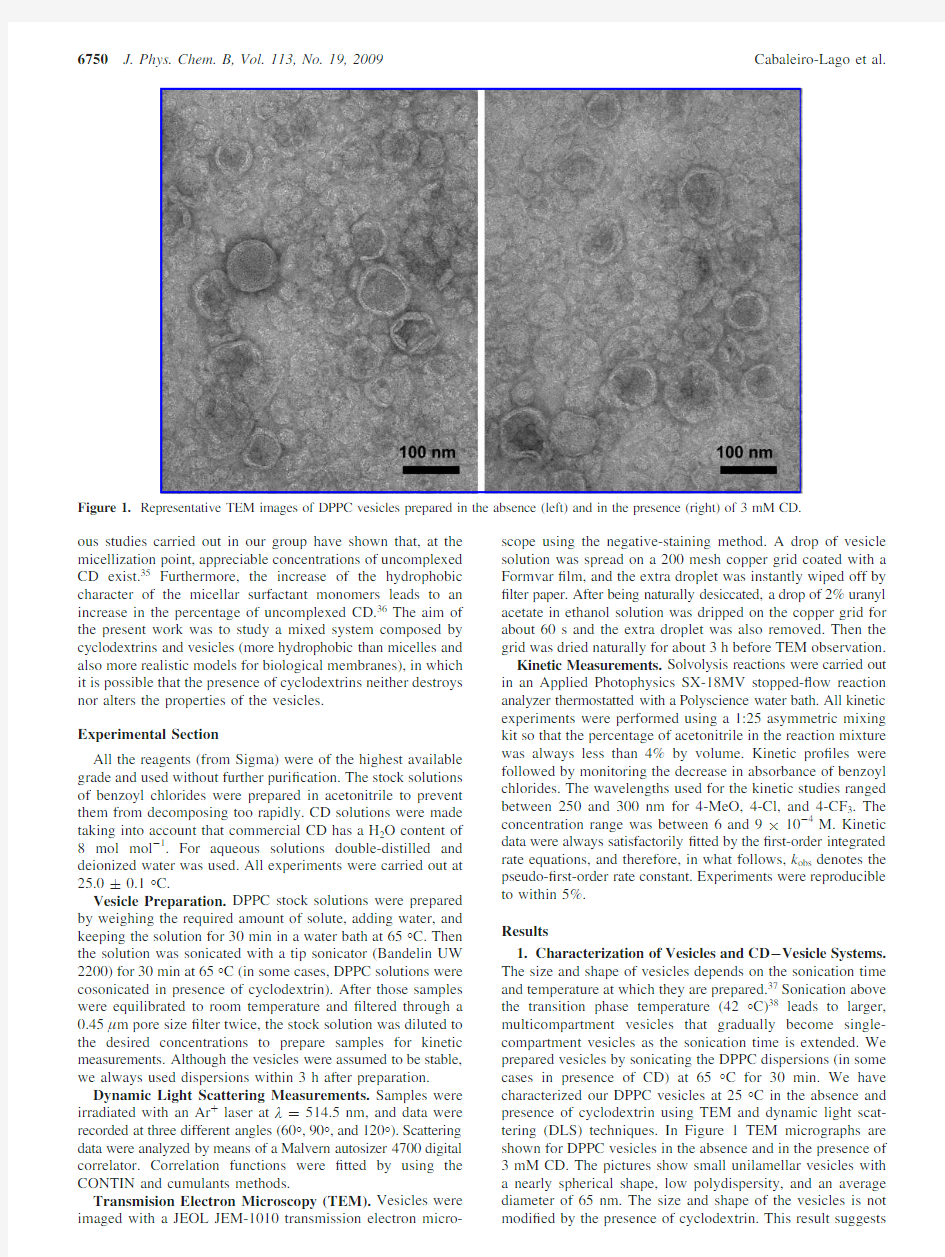
1.Characterization of Vesicles and CD -Vesicle Systems.The size and shape of vesicles depends on the sonication time and temperature at which they are prepared.37Sonication above the transition phase temperature (42°C)38leads to larger,multicompartment vesicles that gradually become single-compartment vesicles as the sonication time is extended.We prepared vesicles by sonicating the DPPC dispersions (in some cases in presence of CD)at 65°C for 30min.We have characterized our DPPC vesicles at 25°C in the absence and presence of cyclodextrin using TEM and dynamic light scat-tering (DLS)techniques.In Figure 1TEM micrographs are shown for DPPC vesicles in the absence and in the presence of 3mM CD.The pictures show small unilamellar vesicles with a nearly spherical shape,low polydispersity,and an average diameter of 65nm.The size and shape of the vesicles is not modi?ed by the presence of cyclodextrin.This result
suggests
Figure 1.Representative TEM images of DPPC vesicles prepared in the absence (left)and in the presence (right)of 3mM CD.
6750J.Phys.Chem.B,Vol.113,No.19,2009Cabaleiro-Lago et
al.
to us the coexistence of vesicles and CD without mutual interactions that lead to destroy the vesicular aggregates.We have also determined the size of the vesicles from DLS measurements.A hydrodynamic diameter of D h )69(5nm was obtained,in good agreement with that determined by TEM.The hydrodynamic diameter of the vesicles prepared cosoni-cating DPPC in the presence of cyclodextrin ([CD])3mM)was D h )70(2nm (similar to that in the absence of CD),suggesting again no interactions between DPPC monomers and CDs.
2.In?uence of CD on the Solvolysis of Substituted Benzoyl Chlorides.Although the hydrolysis of benzoyl chlo-rides in the presence of CDs has been recently studied,31we examined the in?uence of the CD concentration on the solvolysis reaction of substituted benzoyl chlorides to ensure good consistency in the evaluations of the experimental results.In the presence of CD,the apolar inner cavity of the CD provides a solubilization site for the benzoyl chloride with a reversible formation of a 1:1inclusion complex,as shown in Scheme 2.From this kinetic scheme we can obtain the following rate equation:
k obs )
k w +k CD K CD [CD]1+K CD [CD]
(1)
where k w and k CD are the rate constants of the solvolysis of the benzoyl chlorides in bulk water and in the inclusion complex with the cyclodextrin.K CD is the equilibrium constant of the cyclodextrin -benzoyl chloride complex.
Depending on the nature of each benzoyl chloride,the kinetic behavior obtained differs (Figure 2),and this is due to the different mechanisms whereby the reaction takes place.Benzoyl chlorides with electron-withdrawing groups (4-CF 3)favor an associative mechanism,and therefore the inclusion complex is reactive (Figure 2A).On the other hand,benzoyl chlorides with electron-donating substituents (4-MeO and 4-Cl)favor a dis-sociative mechanism that implies the formation of a nonreactive complex (Figure 2B).31
Equation 1can be ?tted to experimental data giving values in good agreement with literature.31(K CD )200(23M -1and k CD )(9.2(0.3)×10-2s -1for 4-CF 3,K CD )391(15M -1for 4-MeO,and K CD )225(21M -1for 4-Cl.)
3.In?uence of DPPC Vesicles on the Solvolysis of Substituted Benzoyl Chlorides.The study of the solvolysis reaction of substituted benzoyl chlorides in the presence of zwitterionic vesicles of DPPC has been recently carried out in our research group.39
Figure 3shows the in?uence of the vesicular aggregates on the solvolysis reactions for the different benzoyl chlorides.The observed kinetic behavior will depend on the substituent of the
aromatic ring.In general,the kinetic effects of the vesicles on the pseudo-?rst-order rate constant can be analyzed on basis of the pseudophase model,40,41assuming a two-pseudophase system in which the reaction is treated as occurring in both a vesicular pseudophase,representing the DPPC bilayer,and an aqueous pseudophase,representing both the bulk medium and the intravesicular compartment (see Scheme 3).The substrate would be distributed between the two regions,and therefore the reaction can take place in either of them.
The overall reaction rate will be the sum of the rates in both pseudophases.This model leads to the following equation:
k obs )
k w +k ves K V [DPPC]1+K V [DPPC]
(2)
where k ves and k w are the ?rst-order rate constants for the vesicular and aqueous pseudophases,respectively,and K V is the association constant or constant of benzoyl chloride distribu-tion between the two pseudophases.
Table 1shows the values of rate constants in the vesicular interface and in water,as well as the distribution constant for the three substituted benzoyl chlorides studied obtained from the ?t of eq 2to experimental data.
4.Solvolysis of Substituted Benzoyl Chlorides in the Presence of the Mixed CD -Vesicle System.To study the CD -vesicles mixed system we carried out sets of experiments in which the [CD]was kept constant,and we observed the effect of increasing the vesicles concentration.
4.1.Sol W olysis of 4-MeO and 4-Cl Benzoyl Chlorides.To study the effect of DPPC vesicles on the solvolysis of 4-MeO benzoyl chloride containing CD,we conducted experiments at constant CD concentration (1.0×10-3,3.0×10-3,
5.0×10-3,and 9.0×10-3M)and variable DPPC concentrations (see Figure 3A).In all cases,the reaction rate decreased with increasing DPPC vesicles concentration.The k obs values obtain-ing by extrapolating to a zero DPPC concentration at each CD concentration are consistent with the k obs values obtained in the presence of CD and in the absence of DPPC (see Figure 2B).The decrease in k obs with increasing [CD]is due to the formation of a nonreactive complex between CD and 4-MeO benzoyl chloride,as we mentioned in section 2.Benzoyl chlorides with electron-donating substituents favor a dissociative pathway in which the departure of the leaving group is the slow step of the reaction.32Taking into account that the solvation ability of the interior of the cyclodextrin is minimal,16,17,42it is expected a negligible or very low reactivity of the inclusion complex formed between these benzoyl chlorides and the CD,then an increase in CD concentration will lead to a decrease in the reaction rate.At a constant [CD],an inhibitory effect of DPPC vesicles on solvolysis of 4-MeO benzoyl chloride is observed.Vesicular and substituent effects can be explained in terms of a duality of reaction paths (associative and dissociative).The dissociative mechanism is strongly affected by the properties of the medium.32,43Vesicles provide a more apolar medium for solvolysis of benzoyl chlorides,with a lower ability to solvate the leaving group,the slow step of the reaction.The inhibition observed in Figure 3A can be attributed to the association of the substrates to the vesicles.The association prevents the access of the substrate to the bulk water reducing the observed reaction rate.Although reaction in the vesicular pseudophase is taking place,the rate constant (k ves )is much smaller than that in water.39In Figure 3A also it can be also observed that the inhibitory effect of vesicles is reduced as the concentration of CD increases due to the competitive association of 4-MeO benzoyl chloride
SCHEME
2
Mixed Vesicle -Cyclodextrin Systems
J.Phys.Chem.B,Vol.113,No.19,2009
6751
to the CD that reduces the amount of substrate available to reacts in the vesicular interface.
Similar results were obtained in the study of the in?uence of DPPC vesicles on the solvolysis of 4-Cl benzoyl chloride containing CD (see Figure 3B).The observed rate constant decreases as the vesicle concentration increases.As for the
4-MeO substituent,the solvolysis of 4-Cl benzoyl chloride occurs through the dissociative channel,with the departure of the leaving group as rate-limiting step.This behavior,as occurs in the absence of cyclodextrin,is due to the association of the substrate to the vesicles and the smaller reactivity in the vesicular interface than in water,leading to a decrease in the reaction rate.We mentioned in section 2that 4-Cl benzoyl chloride forms a nonreactive complex with cyclodextrin;therefore,the observed rate constant in presence of the mixed system CD -vesicles is smaller than in presence of DPPC vesicles.
4.2.Sol W olysis of 4-CF 3Benzoyl Chloride.Figure 3C shows the in?uence of vesicle concentration on k obs for the solvolysis of 4-CF 3benzoyl chloride in the presence of a constant concentration of CD,[CD])1×10-3M.The observed
rate
Figure 2.In?uence of cyclodextrin concentration on k obs for (A)solvolysis of 4-CF 3benzoyl chloride and (B)4-MeO benzoyl chloride,(O )in the absence of DPPC vesicles and (b )in the presence of [DPPC])5×10-4
M.
Figure 3.(A)In?uence of DPPC concentration on k obs for solvolysis of 4-MeO benzoyl chloride in the presence of different cyclodextrin concentrations:(O )0.00,(b )1×10-3,(0)3×10-3,(9)5×10-3,and (?)9×10-3M.(B)In?uence of DPPC concentration on observed rate constant for solvolysis of 4-Cl benzoyl chloride in (O )absence and (b )[CD])1×10-3M.(C)In?uence of DPPC concentration on k obs for solvolysis of 4-CF 3benzoyl chloride in (O )absence and (b )[CD])1×10-3M.
SCHEME
3
TABLE 1:Percentage of the Free Cyclodextrin and Results of Fitting Eq 3or 4to the Experimental Data for the Solvolysis of Benzoyl Chlorides in CD -Vesicle Mixed
Systems Keeping Constant the Cyclodextrin Concentration a
substrate [CD]/mM
%[CD]f k ves /s -1b K V /M -1b 4-MeO 0335(74-MeO 1100(4300(134-MeO 399(3342(134-MeO c 396(3317(144-MeO 5102(4304(214-MeO 9109(12292(514-Cl 00.064(0.005518(404-Cl 1105(140.053(0.007445(554-CF 300.16(0.01107(174-CF 3
1
99(38
0.16(0.03
102(34
a
k w )57s -1(4-MeO),k w )0.195s -1(4-Cl),k w )0.037s -1(4-CF 3),k CD )0.092s -1(4-CF 3),K CD )391M -1(4-MeO),K CD )225M -1(4-Cl),K CD )200M -1(4-CF 3).b Values obtained from ?tting eqs 3and 4to the experimental data.c DPPC and CD were cosonicated,vide infra.
6752J.Phys.Chem.B,Vol.113,No.19,2009Cabaleiro-Lago et
al.
constant,k obs,increases with increasing DPPC vesicles concen-tration,as occurs in the absence of cyclodextrin.Solvolysis of benzoyl chlorides with electron-withdrawing groups(as4-CF3) goes through an associative channel,with the formation of a tetrahedral intermediate that develops a negative charge as rate-limiting step.32The observed catalysis in Figure3C is because of the enhanced stabilization of the associative intermediate due to favorable interactions of cationic head groups of the DPPC vesicles with the developing negative charge at the reaction center.39The presence of CD in the mixed system also leads to an increase in the reaction rate due to the formation of a reactive 1:1complex between CD and4-CF3benzoyl chloride.The primary hydroxyl groups of the cyclodextrin are nucleophilic and react with benzoyl chlorides with electron-withdrawing groups.It can be observed that the value of the observed rate constant extrapolated to zero DPPC concentration is in agree-ment with the values obtained in the absence of vesicular surfactant(see Figure2A).
Discussion
A quantitative interpretation of the experimental behavior observed can be carried out by means of the formalism of the pseudophase model.In the solvolysis of substituted benzoyl chlorides we must consider the existence of three simultaneous reaction paths:the reaction of the free substrate in aqueous medium,the reaction of the complexed substrate with the CD, and the reaction of the substrate in the vesicular surface(see Scheme3).
On the basis of this mechanism we can obtain the following general expression for the observed rate constant:
k obs )
k
w
+k
ves
K
V
[DPPC]+k
CD
K
CD
[CD]
f
1+K
V
[DPPC]+K
CD
[CD]
f
(3)
where[CD]f is the concentration of uncomplexed cyclodextrin that is available to form an inclusion complex with the benzoyl chloride.
In order to apply this kinetic model to our system we need to know the concentration of free cyclodextrin([CD]f)present in the system.Our group has developed a kinetic model that accounts for reactivity in mixed micellar surfactant-CD sys-tems,35allowing us to indicate some characteristics of mixed CD-surfactant systems:(i)for surfactant concentrations lower than the micellization point complexation equilibrium between the surfactant and the cyclodextrin is established.As the surfactant concentration increases we reach a situation in which the concentration of uncomplexed surfactant monomers in equilibrium with the CD is enough for the micellization process to begin.(ii)At the micellization point an appreciable concen-tration of uncomplexed CD exists.36This conclusion was contradictory with the traditional view44that considers that only when all the available cavities of the CD are occupied,the monomers can aggregate to form the micelles,and(iii)the results obtained con?rm that the percentage of uncomplexed CD increases with the hydrophobic character of the surfactant. Therefore,by modulating the hydrophobicity of cationic sur-factants we have found changes in the percentage of uncom-plexed CD in equilibrium with the micellar system between5% and30%.45Moreover,using nonionic surfactant with lower critical micelle concentration(cmc)than cationic ones,46the percentage of uncomplexed CD can increase to almost93%. DPPC vesicles supply a more hydrophobic environment than micelles due to the much lower critical vesicle concentration, as compared to the cmc.Then,we would be able to obtain a vesicle-CD mixed system in which all cyclodextrin would be free and available to bind the organic substrate,as we propose in Scheme3.It is well-known that the addition of CDs to micellar systems produces changes in its physicochemical properties,due to the formation of inclusion complexes CD-surfactant monomer,and if the[CD]is high enough,this complexation process can lead to destroy the micellar ag-gregates.To take this interaction into account,the concentration of free or uncomplexed CD(CD not associated to surfactant molecules)was calculated by using the information obtained from the experiments in systems where the benzoyl chlorides are in presence of a single association entity,CD or vesicles. As pointed out before for the substituted benzoyl chlorides with dissociative mechanism,4-MeO and4-Cl,the reactivity of the substrate complexed with the cyclodextrin is negligible, and then in these cases,eq3can be rewritten as
k
obs
)
k
w
+k
ves
K
V
[DPPC]
1+K
V
[DPPC]+K
CD
[CD]
f
(4)
For any vesicle concentration it is possible to obtain the concentration of free cyclodextrin([CD]f),from the following equation derived from eq4.
[CD]
f
)
k
w
-k
obs
+K
V
(k
ves
-k
obs
)[DPPC]
k
obs
K
CD
(5)
The combination of the curves for the systems with only CD or DPPC(Figure2B and Figure3,parts A and B)can be used as calibration curves and let us obtain the concentration of uncomplexed cyclodextrin in the mixed system formed by CD and a vesicular surfactant.This requires that we assume that the kinetics and equilibrium constants in the individual system are not modi?ed in the mixed system.For each constant concentration of CD of Figure3,parts A and B,we can obtain the value[CD]f from the k obs value of each DPPC concentration. In Table1are shown the obtained mean values of[CD]f.The percentages of free cyclodextrin are compatible with100%and are independent of the cyclodextrin concentration of the medium. This result is in accordance with previous results obtained in our research group45,46that showed that the percentage of uncomplexed CD increases with the hydrophobic character of the micellar surfactant.DPPC provides a more hydrophobic environment than micelles,or at least its critical vesicle concentration is lower than the cmc of micelles.
On the other hand for the4-CF3-substituted benzoyl chloride (with and associative mechanism and therefore a reactive CD-substrate inclusion complex)it is possible to calculate the uncomplexed or free cyclodextrin concentration from eq6, which is obtained from eq3.
[CD]
f
)
k
w
-k
obs
+K
V
(k
ves
-k
obs
)[DPPC]
k
obs
K
CD
-k
CD
K
CD
(6)
The Figure2A values(in absence of DPPC)and Figure3C (in absence of CD)were used as calibration curves and allow us to obtain the concentration of uncomplexed cyclodextrin.As before,the percentage of free cyclodextrin in the mixed system is compatible with100%(see Table1).
The experimental data together with the percentages of free cyclodextrin obtained from eqs5and6allow us to propose the lack of interaction between cyclodextrin and the vesicular surfactant.Therefore,the concentration of DPPC in eqs3and 4corresponds to the total concentration of vesicular surfactant (we are taking into account the very low critical aggregation concentration of vesicles),47and[CD]f is the total cyclodextrin concentration which is available to react with benzoyl chlorides.
Mixed Vesicle-Cyclodextrin Systems J.Phys.Chem.B,Vol.113,No.19,20096753
Taking into account the above considerations(that is,the DPPC and CD f concentrations correspond to the total concentra-tions)eqs4and3can be?tted to the experimental data for the 4-MeO/4-Cl and4-CF3,respectively.The curves traced in Figure 3,parts A and B,correspond with the?t of eq4to the experimental values of k obs.In the case of Figure3C we?t eq 3to the observed rate constant.To simplify the?tting procedure, we have only optimized the parameters corresponding with the reaction in the vesicular pseudophase,k ves and K V(using the values for k w,k CD,and K CD obtained in the absence of vesicles). The value of k ves for the solvolysis of4-MeO is much smaller than that in water and compatible with zero.For this reason and to make easier the?tting procedure we have considered these values negligible in the CD-vesicle mixed system.In Table1are shown the obtained?tting parameters,and es-sentially these parameters are independent of[CD]and agree satisfactorily with the values obtained in the absence of CD. These results indicate the validity of the model being applied. Besides,the constancy in the K V values indicates to us that the properties of DPPC vesicles themselves are not affected by the presence of CD in the medium.
In order to check the validity of the proposed model,we study the effect CD concentration on the solvolysis of substituted benzoyl chloride in the presence of a constant concentration of vesicles,[DPPC])5×10-4M.Figure2A shows the results for4-CF3benzoyl chloride.The observed rate constant increased with increasing CD concentration,as happened in the absence of vesicles,due to the formation of a reactive complex between benzoyl chloride with electron-withdrawing groups and CD.The increase in k obs values in the presence of DPPC vesicles is due the fact of4-CF3benzoyl chloride reacts through an associative channel,which is favored by the vesicles,39as we mentioned in the Results section.Figure2B shows the effect of increasing [CD]on k obs for the solvolysis of4-MeO in the presence of vesicles.The reaction rate shows inhibition behavior with increasing[CD],due the formation of an unreactive inclusion complex between benzoyl chloride with electron-donating groups and CD.The reaction rate decreases in the presence of DPPC vesicles,which provide a more apolar medium,leading to an inhibition of reaction that goes through the dissociative mechanism,as was commented in the Results section.Similar results were found to4-Cl benzoyl chloride(see the Supporting Information).The solid lines in Figure2,parts A and B, represent the best?t of eqs3and4,respectively,to the experimental data.In this case,we optimized the parameters corresponding with the reaction in the presence of cyclodextrins, k CD and K CD(and using the values for k w,k ves,and K V obtained in the absence of cyclodextrin).In Table2are shown the optimized values for these parameters in cyclodextrin and mixed CD-vesicle systems.As we can observe there is a good agreement between the values of k CD and K CD obtained in the absence and presence of DPPC vesicles.These results support the proposed model.
The results obtained and shown in Tables1and2let us conclude that(a)the properties of DPPC vesicles themselves are not affected by the presence of CD in the medium and(b) there is no type of interaction between the CD and the vesicular surfactant monomers,and then all cyclodextrin is present in the mixed system as uncomplexed cyclodextrin.
In our model we considered that properties of DPPC vesicles themselves were not affected by the presence of CD in the medium.The characterization of the mixed system and the kinetic result obtained supports this assumption.Previous kinetic studies have shown that there is no interaction between CD and sodium dodecyl sulfate(SDS)or tetradecyltrimethylammonium bromide(TTABr)micelles.48,49In the other hand,the results obtained from the study of the enthalpy of transfer of cyclo-dextrin from water to the aqueous surfactant solutions suggest the existence of interactions between micelles and cyclodextrins by using?uorinated alkanoates.50However,the existence of these interactions has been questioned recently,51using the self-diffusion NMR technique to study the host-guest interactions between CD and micelles of cationic,anionic,and nonionic surfactants.
As we mentioned,previous studies carried out in our group and by others52showed that,rather than being two competitive processes,the association to the cyclodextrin and the autoas-sociation of surfactant are simultaneous processes,and from the competition between them derives the existence of free cyclodextrin.Our results show that an increase in the hydrophobic-character of the surfactant favors more the autoassociation45,46 rather the association to the cyclodextrin.With the use of a provesicular surfactant,it is possible to obtain a surfactant-CD mixed system in which all cyclodextrin is free and available to react with the organic substrate.Another explanation for the absence of interactions between DPPC vesicles and cyclodex-trins is the high robustness of the vesicles comparing to micelles. Although there is a surfactant concentration threshold below which vesicles do not form,47this threshold is not the result of a dynamic equilibrium between free and vesicular surfactant, like the cmc of micellar media.Once formed,vesicles are not destroyed by dilution.The stability of the vesicles is,in this sense,higher than the micelles.
As a last con?rmation of our results,we have studied the in?uence of DPPC on solvolysis of4-MeO in mixed systems of CD-vesicle,but in this case the vesicular surfactant was cosonicated in presence of[CD])3×10-3M.The hydro-dynamic diameter of the vesicles prepared in this way was D h )70(2nm(similar to that in the absence of CD).The values of k obs obtained increasing the vesicles concentration were similar(see the Supporting Information)to that obtained,at the same starting reactant concentrations,in the mixed system in which the cyclodextrin is added to the previous formed vesicles, leading to a similar values of K V(see Table1).These results reassert our assumption of there is no type of interaction between the CD and the vesicular surfactant monomers.Opposite to what happens with micellar surfactant,the additions of cyclodextrin to the vesicular systems neither destroy nor alter the properties of the vesicles.The results summarized in this work highlight the complexity of CD-vesicle systems,and we consider them of great importance since cyclodextrins and DPPC(or others surfactants)aggregates are potential drug complexing agents. Therefore,formulations that contain both species should take
TABLE2:Results of Fitting Eq3or4to the Experimental
Data for the Solvolysis of Benzoyl Chlorides in CD-Vesicle
Mixed Systems Keeping Constant the DPPC Concentration a
substrate[DPPC]/M k CD/s-1K CD/M-1
4-MeO0391(15
4-MeO5×10-4411(7
4-Cl0225(2
4-Cl5×10-4212(11
4-CF300.092(0.003200(23
4-CF35×10-40.085(0.006268(85
a k w)57s-1(4-MeO),k w)0.195s-1(4-Cl),k w)0.037s-1
(4-CF3),k ves)0.064s-1(4-Cl),k ves)0.16s-1(4-CF3),K v)335
M-1(4-MeO),K v)518M-1(4-Cl),K CD)107M-1(4-CF3).
6754J.Phys.Chem.B,Vol.113,No.19,2009Cabaleiro-Lago et al.
into deep consideration the possible interaction between both species and the consequences on this interaction on its function. Conclusions
A study has been carried out on the solvolysis of substituted benzoyl chlorides in cyclodextrins-DPPC vesicle mixed sys-tems.The reaction takes place simultaneously through dissocia-tive and associative mechanisms.A quantitative interpretation of the experimental behavior observed can be carried out by means of the formalism of the pseudophase model,which allowed us to obtain the thermodynamic and kinetic coef?cients characteristic of the reaction.
The kinetic proposed model lets us determine the percentages of uncomplexed cyclodextrin in equilibrium with the vesicular system which are compatible with100%and are independent of the cyclodextrin concentration in the medium.Transmission electron microscopy and DLS measurements showed that the size and shape of the vesicles are not modi?ed by the pres-ence of cyclodextrin.The results obtained let us conclude that the properties of DPPC vesicles themselves are not affected by the presence of CD in the medium,and there is no type of interaction between the CD and the vesicular surfactant mono-mers,and then all cyclodextrin is present in the mixed system as uncomplexed cyclodextrin.Opposite to what happens with micellar surfactant,the addition of cyclodextrin to the vesicular system neither destroys nor alters the properties of the vesicles. Acknowledgment.Financial support from the Ministerio de Educacio′n y Ciencia(Project CTQ2007-64758),Programa de Recursos Humanos do Plan Galego de Innovacions,Desen-volvemento e Teccnoloxia-INCITE Isidro Parga Pondal(C.C.-L.),and Programa Nacional de Contratacio′n e Incorporacio′n de Recursos Humanos de Investigacio′n,Subprograma Ramo′n y Cajal(J.P.-J.)is acknowledged.
Supporting Information Available:Graphs for the sol-volysis of4-Cl in the presence of CD and solvolysis of4-MeO in the cosonicated CD-vesicles system.This material is available free of charge via the Internet at https://www.wendangku.net/doc/1d9808691.html,. References and Notes
(1)Fendler,J.H.Membrane Mimetic Chemistry;Wiley:New York, 1982.
(2)Lasic,D.D.Liposomes.From Physics to Applications;Elsevier: Amsterdam The Netherlands,1993.
(3)Lian,T.;Ho,R.J.J.Pharm.Sci.2001,90,667–680.
(4)Torchilin,V.P.;Rammohan,R.;Weissig,V.V.;Levchenko,V. Proc.Natl.Acad.Sci.U.S.A.2001,98,8786–8791.
(5)Vodovozova,E.L.;Moiseeva,E.V.;Grechko,G.K.;Gayenko,
G.P.;Nifant’ev,N.E.;Bovin,N.V.;Molotlovsky,J.G.Eur.J.Cancer 2000,36,942–949.
(6)Faroux-Corlay,B.;Clary,L.;Gadras,C.;Hammache,D.;Greiner, J.;Santaella,C.;Aubertin,A.M.;Vierling,P.;Fantini,J.Carbohydr.Res. 2000,327,223–260.
(7)Fendler,J.H.Acc.Chem.Res.1980,13,7–13.
(8)Moss,R.A.;Swarup,S.;Zhang,H.J.Am.Chem.Soc.1988,110, 2914–2919.
(9)Herve′s,P.;Leis,J.R.;Mejuto,J.C.;Pe′rez-Juste,https://www.wendangku.net/doc/1d9808691.html,ngmuir1997, 13,6633–6637.
(10)Carmona-Ribeiro,A.M.;Yoshida,L.S.;Sesso,A.;Chaimovich,
H.J.Colloid Interface Sci.1984,100,433–443.
(11)Garc?′a-R?′o,L.;Herve′s,P.;Mejuto,J.C.;Pe′rez-Juste,J.;Rodr?′guez-Dafonte,P.New J.Chem.2003,27,372–380.
(12)Perez-Juste,J.;Hollfelder,F.;Kirby,A.J.;Engberts,J.B.F.N. Org.Lett.2000,2,127–130.
(13)Klijn,J.E.;Engberts,J.B.F.N.J.Am.Chem.Soc.2003,125, 1825–1833.
(14)Klijn,J.E.;Engberts,https://www.wendangku.net/doc/1d9808691.html,.Biomol.Chem.2004,2, 1789–1799.
(15)Klijn,J.E.;Engberts,https://www.wendangku.net/doc/1d9808691.html,ngmuir2005,21,9809–9817.
(16)Connors,K.A.Chem.Re V.1997,97,1325–1357.
(17)Rekharsky,M.V.;Inoue,Y.Chem.Re V.1998,98,1875–1917.
(18)Schneider,H.J.;Hacket,F.;Ruediger,V.;Ikeda,H.Chem.Re V. 1998,98,1755–1785.
(19)Parrotlopez,H.P.;Ling,C.C.;Zhang,P.;Baszkin,A.;Albrecht,
G.;Derango,C.;Coleman,A.W.J.Am.Chem.Soc.1992,114,5479–5480.
(20)Schalchli,A.;Benattar,J.J.;Tchoreff,P.;Zhang,P.;Coleman,
https://www.wendangku.net/doc/1d9808691.html,ngmuir1993,9,1968–1970.
(21)Ravoo,B.J.;Darcy,R.Angew.Chem.,Int.Ed.2000,39,4324–4326.
(22)Uekama,K.;Hirayama,F.;Irie,T.Chem.Re V.1998,98,2045–2076.
(23)Szejtli,J.Chem.Re V.1998,98,1743–1753.
(24)D’Souza,V.T.;Lipkowitz,K.B.Chem.Re V.1998,98,1741–1742.
(25)Monti,S.;Sortino,S.Chem.Soc.Re V.2002,31,287–300.
(26)Bosca′,F.;Marin,M.L.;Miranda,M.A.Photochem.Photobiol. 2001,74,637–655.
(27)De Guidi,G.;Condorelli,G.;Giuffrida,S.;Puglisi,G.;Giammona,
G.J.Inclusion Phenom.Mol.Recognit.Chem.1993,15,43–58.
(28)Sortino,S.;Scaiano,J.C.;De Guidi,G.;Monti,S.Photochem. Photobiol.1999,70,549–556.
(29)Partyka,M.;Au,B.H.;Evans,C.H.J.Photochem.Photobiol.,A 2001,140,67–74.
(30)Stella,V.J.;Rao,V.M.;Zabbou,E.A.;Zia,V.Ad V.Drug Deli V ery Re V.1999,36,3–16.
(31)Báscuas,J.;Garc?′a-R?′o,L.;Leis,https://www.wendangku.net/doc/1d9808691.html,.Biomol.Chem.2004, 2,1186–1193.
(32)Song,B.D.;Jencks,W.P.J.Am.Chem.Soc.1989,111,8470–8479.
(33)Garcia-Rio,L.;Leis,J.R.;Moreira,J.A.J.Am.Chem.Soc.2000, 122,10325–10334.
(34)Cabaleiro-Lago,C.;Garc?′a-R?′o,L.;Herve′s,P.;Pe′rez-Juste,J.J. Phys.Chem.B2005,109,22614–22622.
(35)Alvarez,A.R.;Garc?′a-R?′o,L.;Herve′s,P.;Leis,J.R.;Mejuto, J.C.;Pe′rez-Juste,https://www.wendangku.net/doc/1d9808691.html,ngmuir1999,15,8368–8375.
(36)Dorrego,B.;Garc?′a-R?′o,L.;Herve′s,P.;Leis,J.R.;Mejuto,J.C.; Pe′rez-Juste,J.Angew.Chem.,Int.Ed.2000,39,2945–2948.
(37)Carmona-Ribeiro,A.M.Chem.Soc.Re V.1992,21,209–214.
(38)Walde,P.;Ichikawa,S.Biomol.Eng.2001,18,143–177.
(39)Cabaleiro-Lago,C.;Garc?′a-R?′o,L.;Herve′s,P.;Pe′rez-Juste,J.J. Phys.Chem.B2006,110,8524–8530.
(40)Romsted,L.S.Surfactants in Solution;Plenum Press:New York, 1984.
(41)Bunton,C.A.;Savelli,G.Ad https://www.wendangku.net/doc/1d9808691.html,.Chem.1986,22,213–309.
(42)Hamai,S.J.Phys.Chem.1990,94,2595–2600.
(43)Bentley,T.W.;Harris,H.C.J.Chem.Soc.,Perkin Trans.21986, 619–624.
(44)Gonzalez-Gaitano,G.;Sanz-Garcia,T.;Tardajos,https://www.wendangku.net/doc/1d9808691.html,ngmuir 1999,15,7963–7972.
(45)Dorrego,B.;Garc?′a-R?′o,L.;Herve′s,P.;Leis,J.R.;Mejuto,J.C.; Pe′rez-Juste,J.J.Phys.Chem.B2001,105,4912–4920.
(46)Cabaleiro-Lago,C.;Garc?′a-R?′o,L.;Herve′s,P.;Mejuto,J.C.;Pe′rez-Juste,J.J.Phys.Chem.B2006,110,15831–15838.
(47)Kawamuro,M.K.;Chaimovich,H.;Abuin,E.B.;Lissi,E.A.; Cuccovia,I.M.J.Phys.Chem.1991,95,1458–1463.
(48)Garc?′a-R?′o,L.;Leis,J.R.;Mejuto,J.C.;Pe′rez-Juste,J.J.Phys. Chem.B1997,101,7383–7389.
(49)Garc?′a-R?′o,L.;Leis,J.R.;Mejuto,J.C.;Pe′rez-Juste,J.J.Phys. Chem.B1998,102,4581–4587.
(50)De Lisi,R.;Milioto,S.;Muratore,N.J.Phys.Chem.B2002,106, 8944–8953.
(51)Cabaleiro-Lago,C.;Nilsson,M.;So¨derman,https://www.wendangku.net/doc/1d9808691.html,ngmuir2005, 21,11637–11644.
(52)Lima,S.;Goodfellow,B.J.;Teixeira-Dias,J.J.C.J.Phys.Chem. B2003,107,14590–14597.
JP901028K
Mixed Vesicle-Cyclodextrin Systems J.Phys.Chem.B,Vol.113,No.19,20096755
c语言试题及答案
《C语言》课程综合复习资料 一、单选题 1. 在C语言中,字符型数据在存中的存储形式是 A)原码 B)补码 C)反码 D)ASCII码 2. 在C语言中,十进制数47可等价地表示为 A) 2f B) 02f C) 57 D) 057 3. 设有定义:int x=12,n=5; 则表达式 x%=(n%2) 的值为 A) 0 B) 1 C) 2 D) 3 4. 设有定义语句:char str[][20]={,"Beijing","中国石油大学"},*p=str; 则printf("%d\n",strlen(p+20)); 输出结果是 A)10 B) 6 C) 0 D) 20 5. 已定义以下函数: fun(int *p) { return *p; } 该函数的返回值是 A)不确定的值 B)形参p所指存储单元中的值 C)形参p中存放的值 D)形参p的地址值 6. C语言中,函数返回值的类型是由 A)return语句中的表达式类型决定 B)调用函数的主调函数类型决定 C)调用函数时的临时类型决定 D)定义函数时所指定的函数类型决定 7. 有以下函数定义: void fun( int n , double x ) { …… } 若以下选项中的变量都已正确定义并赋值,则对函数fun的正确调用语句是 A) fun( int y , double m ); B) k=fun( 10 , 12.5 ); C) fun( 10 , 12.5 ); D) void fun( 10 , 12.5 ); 8. 以下选项中不能正确赋值的是 A) char b[]={′H′,′e′,′l′,′l′,′o′,′!′}; B) char b[10];b="Hello!";
适合娶亲婚礼上唱的歌最全版本
1.《水晶》(新人对唱的) 2.《真想见到你》(李汶的歌,新娘独唱的哦,实力派!) 3.《月亮代表我的心》(比较悠久啦) 4.《深情相拥》(对新人的唱功要求颇高啊) 5.《很爱很爱你》(也是新娘独唱哦,不过改了歌词,例如”看着她走向你,那幅画面多美丽”改成“我现在走向你,那幅画面多美丽”) 6.《第一次》(光良的歌,不用我多说了吧) 7.《love,love,love》(八错,调动现场气氛满灵的,新娘唱的也八错滴好象唱歌的新娘居多,我们的男士们很害羞呢) 8.《明明很爱你》(马来西亚的歌手唱的歌满受JMS的欢迎,大概是曲风比较明快吧) 9.《神话》(成龙大哥的版本比较通俗,八过对MM的要求高些,韩红的版本实力派的JMS挑战一下啊!) 10.《选择》(内敛型的JMS可以考虑的一种) 11.《最浪漫的事》(经典的歌曲,用在婚礼上再合适不过。) 12.《宁静的夏天》(节奏轻快,简洁,个人满喜欢,夏天结婚的可以试试) 13.《牵手》(应该是满老的歌了,有空我去听下再发表意见恩,听了个开头就知道了,应该是满苦情的歌,婚礼上就需要考虑是否适合了额.跟年龄有关吧) 14.《你最珍贵》(又一个对唱功有要求的) 15.《恋爱达人》(利用歌词可以搞些小剧情,效果应该不错。) 16.《恋爱频率》(看来是对流行音乐把握很敏锐的MM,) 17.《我只在乎你》(大家帮忙改改歌词吧) 18.《明明白白我的心》(简单的情歌,不太唱歌的JMS也可以小试身手了) 19.《你是我最深爱的人》(应该是男士发挥的时候了吧) 20.《屋顶》(个人还是喜欢杰伦的版本) 21.《在我生命中的每一天》(对唱的、抒情的慢歌永远是大家的最爱+首选) 22.《小夫妻》(一般用来做背景音乐较多的歌,可能太通俗了些,唱的人八多啊,不过还 是赞一个) 23.《不得不爱》() 24.《爱你一万年》(LG唱给LP听,记住表情,一定要深情!迷倒一大片。。。。。掌声鼓励一下) 25.《让我取暖》(很适合年轻夫妻对唱的情歌,有Young的气息) 26.《明天我要嫁给你了》 27.《你是我的老婆》(好歌啊,好歌。。。,终于又有适合GG们独唱的好歌了。) 28.《大城小爱》() 29.《你是我的幸福吗》(MM们独唱之作,不要害羞,大胆的唱给GG们听吧!) 30.《出嫁》(zhangning_she MM真是需要再表扬一下,提供了这么多歌,而且首首都这么经典,不错的情歌,可对唱) 31.《我愿意》(可独唱,也可双人合唱的佳作) 32.《你是我心底的烙印》(一人一句,配合默契啊) 33.《甜蜜蜜》(邓JJ的怀旧老歌,永远最好听) 34.《北极雪》(旋律很容易上口,不只冬天适合唱,夏天唱可以带来一丝凉意) 35.《被风吹过的夏天》(如果是在夏天相恋的JMS注意了额,这里就有一首适合你们对唱 的歌曲了)
12年高考湖北文言文《家有名士》详细注解家有名士
2012年高考湖北卷 家有名士 1 南朝2 刘义庆 3 ?王湛4 既除所生服5 ,遂停墓所6 。兄王浑7 之子济 8 每来拜墓9,略不过叔10,叔亦不候。济脱时过11,止 12 寒温13而已。后聊14试问近事15,答对甚有音辞16,出 济意外,济极惋愕17。仍与语,转造18清微19 。济先略无20子侄之敬21,既闻其言,不觉懔然22,心形23俱肃24。 遂留共语,弥日累夜25。济虽俊爽26,自视缺然27 ,乃喟然28 叹曰:“家有名士,三十年而不知!” ?济去,叔送至门。济从骑1 有一马,绝.2 难乘,少3 能骑者。济聊4问叔:“好5骑乘不?”曰:“亦好尔。”济 又使骑难乘马,叔姿形6既妙,回策如萦7,名骑8无以 9 过之。济益10叹其11难测,非复12 一事。 ?【邓粲1 《晋纪》曰:“王湛字处冲,太原人。隐德,人莫之知,虽兄弟宗族,亦以为痴,唯父昶异焉。昶丧,居 墓次2,兄子济往省3湛,见床头有《周易》4 ,谓湛曰:‘叔 父用此何为?颇5曾看不?’湛笑曰:‘体中6 不佳时,脱 复看耳7。今日当与汝言。’因8 共谈《易》,剖析入微,济 所未闻,叹不能测9 。 ?济性好马,而所乘马骏驶1 ,意甚爱之。湛曰:‘此虽小驶2,然力薄不堪3 苦。近见督邮马,当胜此,但养不至 耳。’济取督邮马,谷食4 十数日,与湛试之。湛未尝乘马,卒5然便驰骋,步骤不异于济,而马不相胜6 。湛曰:‘今直 行车路7,何以别马胜不8?唯当就.9蚁封10 耳。’于是就蚁封盘马11,果倒踣12,其俊识13天才14乃尔15。”】 ?既还,浑问济:“何以暂行累日1 ?”济曰:“始2 得 一叔。”浑问其故,济具3叹述4如此。浑曰:“何如5 我?”济曰:“济以上人。”武帝每见济,辄以湛调之,曰:“卿家痴叔死未?”济常无以答。既而得叔,后武帝又问如前,济 曰:“臣叔不痴。”称其实美6。帝曰:“谁比7 ?”济曰:“山涛8以下,魏舒9以上。”【《晋阳秋》曰:“济有人伦鉴识10, 见湛,叹服其德宇11 。时人谓湛上方山涛不足,下比魏舒有余。”】 【注】正文选自南朝刘义庆的《世说新语》,【】内的文字是南朝刘孝标的注解。有删改。 ①【名士míng shì】:(1)指已出名而未出仕的人。郑玄注:“名 士,不仕者。”(2)泛指有名的人。杜甫《陪李北海宴历下亭》诗:“海 内此亭古,济南名士多。”(3)特指恃才放达、不拘小节的人。”清 袁赋诚《睢阳尚书袁氏家谱》:“袁可立爱书,不事生产。所与游皆名士”(4)指刑名之士(崇尚法家之士)。《史记·律书》:“自是以后,名士迭兴,晋用咎犯,而齐用王子,吴用孙武,申明军约,赏罚必信。” 【名士】一词,源于我国古代魏晋时期 。魏晋多名士 ,他们的特点: 多隐居,峨冠博带,说怪话但博学多才,形貌潇洒,偶尔也有放浪形骸的。有云:从来圣贤皆寂寞,是真名士自风流 ②【南朝】:南朝(420—公元589,共170年)是东晋之后建 立于南方的四个朝代(宋、齐、梁、陈)的简称。自公元420年刘裕灭亡东晋王朝建立宋,接着是齐、梁、陈,而陈朝最终于589年被隋灭。 它们存在的时间都相对较短,南朝宋(420年—479年,共60年);南朝齐(479年—502年,共24年);南朝梁(502年—557年,共56年);南朝陈(557年—589年,共33年);其中最长的不过60年,最短的仅有24年,是我国历史上朝代更迭较快的一段时间。此时,中国正处于南北分治的时期,在我国历史上南朝与北方政权北魏、东魏、西魏、北齐、北周并称南北朝。 秦汉三国两晋南北朝隋唐五代十国宋元明清 ③【刘义庆】(403—444),字季伯,汉族,原籍南朝宋彭城(今 江苏徐州)人,文学家,南朝刘宋文学家。刘宋武帝刘裕之侄,在诸王 中颇为出色,十分被看重。刘义庆是这本书的编者,并不为作者。《世说新语》是魏晋南北朝时期“志人小说”的代表作。依内容可分为“德行”“言语”“政事”“文学”“方正”等三十六类,每类收有 若干则,全书共一千多则,每则文字长短不一,有的数行,有的三言两语,从此可见笔记小说“随手而记”的诉求及特性。《世说新语》主要记述世人的生活和思想,及统治阶级的情况,反映了魏晋时期文人想言行,和上层社会的生活面貌,记载颇为丰富真实,描述了当时士人所处的时代状况及政治社会环境,展示了“魏晋清谈”的风貌。京尹时期(15-30岁)。刘义庆15岁一路来平步青云,其中任秘书监一职,掌管国家的图书著作,有机会接触与博览皇家典籍,对《世说新语》的编撰奠定了良好的基础,17岁升任尚书左仆射(相当于副宰相),可惜的是,《世说新语》一书刚刚撰成,刘义庆就因病离开扬州,回到京城不久便英年早逝,时年仅41岁,宋文帝哀痛不已,赠其谥号为“康王”。 ④【王湛zhàn 】:(249~295年),字处冲,西晋太原晋阳(今山 西太原)人,官至西晋汝南内史,又称王汝南,王昶之子,王浑之弟,儿子是王承。少有识度,身长七尺八寸,龙额大鼻,少言语。服完父丧后,闭门不交宾客,冲素简淡,沉静和顺。晚成,被同族认为痴,起初只有父亲王昶欣赏他。后来被侄子王济称赏,在应答晋武帝时说王湛人材在“山涛以下,魏舒以上”,由此渐而知名。二十八岁方出仕,历任秦王文学、太子洗马、尚书郎、太子中庶子、汝南内史。元康五年(295年)去世,时为四十七岁。 【湛zhàn 】1、深:精~。~恩(深恩)。~蓝。湛深 深湛;精深 湛深的艺术功力。2. 清澈:清~。澄~。1.湛蓝 zhànlán 晴天的蓝色;湖海等的深蓝色。.湛清 清澈 天空湛清如水。 【冲素chōn ɡ sù】 亦作“冲素”。冲淡纯朴。 【冲淡chōng dàn 】冲和、淡泊,叫做冲淡。 冲淡和纤秾不同。纤秾用的是浓彩,冲淡施的是淡墨。 冲淡并非淡而无味,而是冲而不薄,淡而有味。 魏晋文人濯足清流,不染尘俗,同封建权贵不合作的精神,对安静、美好的理想境界的憧憬,是形成冲淡的一个重要原因。 ⑤【除所生服】:为父母守丧完毕。 【所生suǒ shēng 】: 1.生 身父母。: 注:“所生,指亲母。” 南朝 宋 刘义庆 《世说新语·赏誉》:“ 王汝南既除所生服,遂停墓所。 【除服】:亦称“除丧”、“脱服”。除去、脱去丧服。谓守孝三年完毕。 ⑥【墓所】:1.墓地,坟地、墓次。 【墓 mù】 埋葬死人的地方:墓穴。墓地。墓园。墓道。墓碑。坟墓。墓志铭。 【所 suǒ】 处,地方:住所。哨所。场所。处所。 机关或其他办事的地方的名称:研究所。派出所。 ⑦【王浑】(223-297),字玄冲,太原晋阳(今山西太原)人。三国曹魏后期至西晋初期的大臣,东汉代郡太守王泽之孙,曹魏司空王昶之子。承袭父亲京陵侯之位,属魏大将军曹爽部下。嘉平元年(249),曹爽被杀,王浑随之免职。后来又被起用为怀县(今河南沁阳)县令,参司马昭的安东将军军事,任散骑黄门侍郎、散骑常侍。咸熙年间(264-265),为越骑校尉。王浑曾辅佐晋武帝和晋惠帝两代君主,在晋初的军事和政治上作出了一定贡献。特别是在平吴作战方面功绩显著,因此官职累累升迁。 ⑧【王济】(246~291),字武子,太原晋阳(今山西太原)人,名士。西晋大将军王浑的次子。王济才华横溢,风姿英爽,气盖一时,被晋武帝司马炎选为女婿,配常山公主。王济爱好弓马,勇力超人,又善《易经》、《老子》、《庄子》等。文词俊茂,名于当世,与姐夫和峤及裴楷齐名。王济年四十六岁,先其父王浑而亡,追赠骠骑将军。 ⑨【拜墓】:拜扫坟墓。拜扫baì sǎo :在墓前祭奠;扫墓。《南史·梁 纪中·武帝下》:“拜扫山陵,涕泪所洒,松草变色。” 白雪遗音·马头调·雷峰塔》:“清明拜扫,搭船借伞,前世恩人来相见。”⑨【】又如:略等(大约相等,差不多);略绰(阔大;大略);略订(约略计算);略约(约略) ⑩【略不过叔】:基本上、几乎不拜访他的叔叔。 略lüè本义: 封疆土地;天子经略土地,定城国,制诸侯。——《左传·昭公七年》1、基本上,几乎,稍稍,全,皆,都 敬亭丧失其资略尽。—— 清· 黄宗羲《柳敬亭传》 略无慕艳意。——明· 宋濂《送东阳马生序》2 稍稍,稍微;略懂一点,略识文字(初识文字,认字不多)3通“掠”。抢劫;夺取 “属盗起于境,资产略尽,迫寒馁而无忧叹。”---《郝逢传》4、谋略:móu lüè,雄才大略: xióng cái dà lüè:非常杰出的才智和谋略。才,才能。略,计谋。 ?【脱时tuō】:结合上下文,应做有时、偶尔的意思来讲。偏义副词,重点由“时”字联想。脱字确实不知道作什么讲。 脱tuō 肉去骨曰脱。——《尔雅》 1. 离开,落掉:~产。~发(fà)。~节。~离。~落。~贫(摆脱贫困)。~稿(完成著作)。~手。摆~。挣~。临阵逃~。 2. 遗漏:~漏。~误。~文(因抄刊古书而误脱的字。亦称“夺文”)。3. 取下,除去:~下。~帽。~氧。~脂。~胎换骨。4. 倘若,或许:~有不测。5. 轻慢:~略(放任,不拘束)。~易(轻率,不讲究礼貌)。轻~(轻率,不持重,放荡)。 脱产学习就是参加工作后再去校内进行全日制学习的方式,其管理模式与普通高校一样,学习期间不在原单位工作,不占用周六和周日的工休时间,对学生有正常的、相对固定的授课教室和管理要求,有稳定的寒暑假期安排。 ?【止zh ǐ 】: 1. 仅,只:~有此数。不~一回。2. 停住不动:~ 步。截~。3. 拦阻,使停住:~痛。禁~。4. 古同“趾”,脚;脚趾头。 ? 【寒温hán wēn 】 1、.指问候冷暖起居。 晋 干宝 《搜神记》 卷十六:“忽有客通名诣 瞻 ,寒温毕,聊谈名理。” 元 尚仲贤 《柳毅传书》第三折:“施礼罢,叙寒温。”《红楼梦》第一○五回:“众亲友也有认得 赵堂官 的,见他仰着脸不大理人,只拉着 贾政 的手笑着説了几句寒温的话。” 管桦《将军河》第一部第四一章:“﹝ 董士清 ﹞不自然地呲着牙,满口寒温:‘诸位冷不冷啊?辛苦啦!’”2、冷暖。宋 司马光 《和始平公见寄》诗:“违离詎几时,风色变寒温。”3.中医指两种药性,寒性或温性。 明 李时珍 《本草纲目·序例·神农本经名例》:“药有酸、咸、甘、苦、辛五味,又有寒、热、温、凉四气。”注引 宗奭 曰:“寒、热、温、凉,是药之性。” 鲁迅 《二心集·“好政府主义”》:“因为自三民主义以至无政府主义,无论它性质的寒温如何,所开的究竟还是药名。” ?【聊liáo 】本义:耳鸣 1. 姑且,勉强,凑凑和和:~且(姑且)。~ 以自娱。~复尔尔(姑且如此)。~备一格。2. 依赖,寄托:无~。百 无~赖。3. 略微:~表寸心。4. 闲谈:~天。闲~。5. 耳鸣:~啾。 ?【近事】:近日之事,过去不久的事情。 ?【音辞yīn cí】 1.言谈;辞令。 南朝 宋 刘义庆 《世说新语?赏誉》:“后聊试问近事,答对甚有音辞,出 济 ( 王济 )意外。” 北齐 颜之推 《颜氏家训?勉学》:“以外率多田野閒人,音辞鄙陋,风操蚩拙。”2.文词。 唐 刘知几 《史通?序例》:“ 枚乘 首唱《七发》,加以《七章》、《七辩》,音辞虽异,旨趣皆同。”3.音调歌词。《旧唐书?曹确传》:“ 可及 善音律,尤能转喉为新声,音辞曲折惋愕,听者忘倦。”惋愕 ?【惋愕wǎn è】:怅叹惊愕《梁书·昭明太子统传》:“太子仁德 素著,及薨,朝野惋愕。” 【 惋w ǎn 】: 叹惜,憾恨:~惜。~伤。~叹。悲~。哀~。【愕è】:1. 惊讶:~胎。~异。惊~。错~。闻之~然。 ?【造zào 】:1、到。造访;夜造(深夜前往造访);造府拜瞻(敬辞。 到府上去拜访);造请(前往问候、拜见);造谒(造请);造谢(登门致谢) 2、[学 业等]达到的程度或境界。在本文引申为转至、转到。⒉(学业或技艺)所达到的程度:造~。苦心孤~(指刻苦钻研,达到别人不及的境地)。【造诣zào yì】(1)学业、学问等修炼的成果。如:造诣很深。(2)拜访。《晋书·陶潜传》:“酣醉便反,未尝有所造诣。”(3)泛指足迹所至。 《新唐书·崔咸传》:“咸素有高世志,造诣崭远,闲游终南山,乘月吟啸,至感慨泣下。”【诣yì】 1.到,旧时特指到尊长那里去:~阙。~前请教。 ?【清微qīng wēi 】 1.清淡微妙。明 贾仲名 《金安寿》第一折: “韵清微,高山流水野猿嘶, 楚 雨 湘 云塞雁飞,清风明月孤鹤唳。” 清 恽敬 《<靖节集>书后二》:“其诗清微通澈,雄厉奋发,如其人,如其人焉。” ?【略无)lüè wú】:全无,毫无。《三国志·蜀志·赵云传》“以 云 为翊军将军” 裴松之 注引《赵云别传》:“ 赵云 身自断后,军资什物,略无所弃。”张真 《谈京剧<猎虎记>》:“革命,对每个人都是很大的冒险,而这一些人,却都挺身而出,略无难色。”【略lüè】本义:封疆土地;天子经略土地,定城国,制诸侯。——《左传·昭公七年1、基本上,几乎,稍稍,全,皆,都 敬亭丧失其资略尽。—— 清· 黄宗羲《柳敬亭传》 略无慕艳意。——明· 宋濂《送东阳马生序》 ○ 21【敬】:1、尊敬、恭敬。尊重,有礼貌地对待:尊~。致~。~重(zhòng )。~爱。~仰。恭~。~辞。~慕。~献。2、有礼貌地送 上去:~酒。~香。 ○ 22【懔然lǐn rán 】1.危惧貌;戒惧貌。《荀子·议兵》:“ 紂 刳 比干 ,囚 箕子 ,为炮烙刑;杀戮无时,臣下懍然。” 杨倞 注:“懍然,悚栗之貌。”2.严正貌。 南朝 宋 刘义庆 《世说新语·轻诋》:“ 桓公 懍然作色……四坐既骇,袁亦失色。”杨沫 《青春之歌》第一部第四章:“她咬着嘴唇,懔然地瞪视着这些人。”【懔l ǐn 】◎懔,敬也。——《广雅》,也作 畏惧。懔惧(畏惧);懔畏(畏惧) ○ 23【心形xīn xíng 】: 内心和形体,精神与身体。 南朝 宋 刘义庆 《世说新语·赏誉》:“﹝ 王济 ﹞既闻其言,不觉懔然,心形俱肃。” 唐 白居易 《足疾》诗:“应须学取陶彭泽 ,但委心形任去留。” ○ 24【肃sù】1. 恭敬:~立。~坐。~然。2. 严正,认真:严~。~静。~穆。整~。3. 躬身作揖,迎揖引进:~客。4. 萎缩:~杀。 ○ 25【弥日累夜mí rì lěi yè】 连日连夜,夜以继日。同“夜以继日”。【弥日mí rì】 终日,满日。《后汉书·文苑传下·边让》:“登 瑶臺 以回望兮,冀弥日而消忧。” 李贤 注:“弥,终也。” 南朝 宋 刘义庆 《世说新语·文学》:“ 张 遂诣 刘 …… 真长 延之上坐,清言弥日,因留宿至晓。”清 无名氏 《后会仙记》:“会既散, 仇生 惘然若有所失,悵念弥日。”【弥mí 】 1. 满,遍:~满。~月(a.整一个月;b.婴儿满月)。~望(满眼)。~天(满天,形容极大的)。2. 补,合:~补。~缝。~封。3. 更加:~坚。欲盖~彰。4. 水满的样子:~漫。 5. 久,远:~留(病久留不去,后称病重将死)。~亘(连绵不断)。 【累夜lěi yè】连夜。《晋书·荀晞传》:“终日累夜,不出户庭。”唐 杜甫 《奉赠卢五丈参谋》诗:“説诗能累夜,醉酒或连朝。【累léi 】1. 〔~~〕a.连续成串,如“果实~~”;b.颓丧的样子,如“~~若丧家之犬”。2. 〔~赘〕a.多余,不简洁,如“文字~~”;b.使人感到多余或麻烦的事物,如“负重登高,不胜~~”(“赘”均读轻声)。【累lěi 】 ㄌㄟˇ1. 连续,重叠,堆积:~计。~日。~积。~~。日积月~。连篇~牍。2. 照原数目多少而递增:~进税。 3. 连及,连带:~及。牵~。拖~。” ○ 26【俊爽 jùnshuǎng 】英俊豪爽;人品高超,性格豪爽。风姿俊爽;少而俊爽。1、英俊清朗。《晋书·裴楷传》:“ 楷 风神高迈,容仪俊爽。”《旧唐书·裴度传》:“ 度状貌不踰中人,而风彩俊爽。” 茅盾 《动摇》:“但此时眉尖稍稍挑起,却又是俊爽英勇的气概。” 2、雄健敏捷。 元 辛文房 《唐才子传·耿湋》:“诗文俊爽。” ○ 27【缺然quē rán 】1).有所不足。《庄子·逍遥游》:“吾自视缺然,请致天下。” 成玄英 疏:“自视缺然不足,请将帝位让与贤人。” 唐 司空图《与李生论诗书》:“愚幼常自负,既久而逾觉缺然。” 宋 王安石 《除参知政事谢表》:“承弼之任,贤智所难;顾惟缺然,何以堪此?” 清 方苞 《汉文帝论》:“世徒见其奉身之俭,接下之恭,临民之简,以为 黄 老 之学则然,不知正自视缺然之心之所发耳。” (2).缺失。《晋书·张骏传》:“每患忠言不献,面从背违,吾政教
C语言试题选择题及答案
★第1 题: 阅读程序,选择程序的运行结果___A___。 #include <> main() { int x; x=try(5); printf(“%d\n”, x); } try(int n) { if(n>0) return(n*try(n-2)); else return(1); } A. 15 B. 120 C. 1 D. 前面3个答案均是错误的 第2 题: 在下列结论中,只有一个是正确的,它是___A___。 A. 递归函数中的形式参数是自动变量 B. 递归函数中的形式参数是外部变量 C. 递归函数中的形式参数是静态变量 D. 递归函数中的形式参数可以根据需要自己定义存储类型★第3 题: 阅读程序,选择程序的输出结果__A___。 #include <> f(int x, int y) { return(y-x); } main() { int (*g)(int,int); int a=5, b=6, c=2; g=f; c=(*g)(a,b);
printf(“%d\n”, c); } A. 1 B. 2 C. 3 D. 前面3个答案均是错误的 第4 题: 阅读程序,选择程序的输出结果__D___。#include <> char *p=”abcdefghijklmnopq”; main() { while(*p++!=’e’) ; printf(“%c\n”, *p); } A. c B. d C. e D. f ★第6 题: 阅读程序,选择程序的输出结果___D___。#include <> void prtv(int *x) { printf(”%d\n”, ++*x); } main() { int a=25; prtv(&a); } A. 23 B. 24 C. 25 D. 26 第7 题:
首儿歌大全歌词完整版
因很多家长向麦麦粥铺反映,想要儿歌50首大全的歌词。我们也花了大量的时间和精力,通过手打不断把歌词完善到趋于完整。希望可以让妈妈们和孩子一起在听的同时,更增加一份教学的乐趣。 您的五分好评是我们进步的最大动力,谢谢。 精心整理,真心希望对孩子的培养能有一丝帮助。 【儿歌串烧50首】第1首:《家庭称呼》歌词 爸爸爸爸daddy daddy daddy daddy 妈妈妈妈mami mami mami mami 哥哥弟弟brother brother brother 姐姐妹妹sister sister sister 爷爷爷爷grandpa grandpa grandpa 奶奶奶奶grandma grandma grandma 伯伯叔叔和舅舅 英文全都叫uncle uncle uncle uncle 姑姑婶婶和阿姨 英文全都叫auntie auntie auntie auntie 爸爸爸爸daddy daddy daddy daddy 妈妈妈妈mami mami mami mami 哥哥弟弟brother brother brother 姐姐妹妹sister sister sister 爷爷爷爷grandpa grandpa grandpa 奶奶奶奶grandma grandma grandma 伯伯叔叔和舅舅 英文全都叫uncle uncle uncle uncle 姑姑婶婶和阿姨 英文全都叫auntie auntie auntie auntie 爸爸爸爸daddy daddy daddy daddy 妈妈妈妈mami mami mami mami 哥哥弟弟brother brother brother 姐姐妹妹sister sister sister 爷爷爷爷grandpa grandpa grandpa 奶奶奶奶grandma grandma grandma 伯伯叔叔和舅舅 英文全都叫uncle uncle uncle uncle 姑姑婶婶和阿姨 英文全都叫auntie auntie auntie auntie 【儿歌串烧50首】第2首:《家族歌》歌词 爸爸的爸爸叫什么?爸爸的爸爸叫爷爷; 爸爸的妈妈叫什么?爸爸的妈妈叫奶奶; 爸爸的哥哥叫什么?爸爸的哥哥叫伯伯; 爸爸的弟弟叫什么?爸爸的弟弟叫叔叔; 爸爸的姐妹叫什么?爸爸的姐妹叫姑姑。 妈妈的爸爸叫什么?妈妈的爸爸叫外公; 妈妈的妈妈叫什么?妈妈的妈妈叫外婆;
歌词、
本站歌词来自互联网北极雪下在那头 寂寞不寂寞 谁的想念是他的等候你若问我快不快乐寂寞不寂寞 牵你手贴我手 感觉我的脉搏 你要试着了解 试着体会 用心好好感觉 然后你才能够看得见快乐伤悲 也许我的眼泪 我的笑脸 只是完美的表演 听说北极下了雪 北极雪下在那头 寂寞不寂寞 谁的想念是他的等候你若问我快不快乐寂寞不寂寞 牵你手贴我手 感觉我的脉搏 你要试着了解 试着体会 用心好好感觉 然后你才能够看得见快乐伤悲 也许我的眼泪 我的笑脸 只是完美的表演 听说北极下了雪 你要试着了解 试着体会 用心好好感觉 然后你才能够看得见快乐伤悲 也许我的眼泪 我的笑脸 只是完美的表演 听说北极下了雪 你要试着了解 试着体会
用心好好感觉 然后你才能够看得见 快乐伤悲 也许我的眼泪 我的笑脸 只是完美的表演 听说北极下了雪 本站歌词来自互联网 本站歌词来自互联网 想唱就唱 演唱:张含韵 推开夜的天窗 对流星说愿望 给我一双翅膀 能够接近太阳 我学着一个人成长 爱给我能量 梦想是神奇的营养 催促我开放 想唱就唱 要唱的响亮 就算没有人为我鼓掌 至少我还能够勇敢的自我欣赏想唱就唱 要唱的漂亮 就算这舞台多空旷 总有一天能看到挥舞的荧光棒想唱就唱 演唱:张含韵 推开夜的天窗 对流星说愿望 给我一双翅膀 能够接近太阳 我学着一个人成长 爱给我能量 梦想是神奇的营养 催促我开放 想唱就唱 要唱的响亮 就算没有人为我鼓掌 至少我还能够勇敢的自我欣赏想唱就唱 要唱的漂亮 就算这舞台多空旷
总有一天能看到挥舞的荧光棒想唱就唱 要唱的响亮 至少我还能够勇敢的自我欣赏就算没有人为我鼓掌 想唱就唱 要唱的漂亮 就算这舞台多空旷 总有一天能看到挥舞的荧光棒想唱就唱 本站歌词来自互联网 本站歌词来自互联网 弦子- 沿海地带 空荡荡的月台 入秋微凉的海 微风把脚下的树叶都吹开 火车就要出发催促我快离开 我的心已超载 你不了解的爱 当失望逐渐将一切都掩埋 没想到害怕更真实存在 在沿海地带放逐我的爱 孤单也很精采 我相信我们都有该去的未来 不该在原地徘徊 MUSIC 我其实很明白 梦醒了就不在 只是还挣扎着不让他离开 紧紧抓着的也都是空白 在沿海地带放逐我的爱 孤单也很精采 我相信我们都有该去的未来 不该在原地徘徊 在沿海地带我远远离开 要更自由自在 不要我的心随着大厅的钟摆 停留在原地感慨 本站歌词来自互联网
JAVA语言程序设计期末考试试题及答案
1234124JAVA语言程序设计考试试题及部分答案 一、单选题:(每题1分)下列各题A)、B)、C)、D)四个选项中,只有一个选项是正确的,请将正确选项的标记写在题干后的括号内。 1.下列语句序列执行后,k 的值是( B ) 。 int m=3, n=6, k=0; while( (m++) < ( -- n) ) ++k; A)0 B) 1 C) 2 D) 3 2.设i 、j 为int 型变量名, a 为int 型数组名,以下选项中,正确的赋值语句是( B ) 。 A)i = i + 2 B) a[0] = 7; C) i++ - --j; D) a(0) = 66; 3.Java语言的类间的继承关系是(B )。 A)多重的B) 单重的C) 线程的D) 不能继承 4.设有定义int i = 6 ; ,则执行以下语句后,i 的值为( C ) 。 i += i - 1; A) 10 B) 121 C) 11 D) 100 5.下列选项中,用于在定义子类时声明父类名的关键字是( C ) 。 A) interface B) package C) extends D) class 6.若已定义byte[ ] x= {11,22,33,-66} ; 其中O W k<3,则对x数组元素错误的引用是(C )。 A) x[5-3] B) x[k] C) x[k+5] D) x[0] 7.下列语句序列执行后, ch1 的值是( B ) 。 char ch1='A',ch2='W'; if(ch1 + 2 < ch2 ) ++ch1; A) 'A' B) 'B' C) 'C' D) B
味道歌词解析
10 味道 作词:姚谦 作曲:黄国伦 演唱:张学友 专辑:张学友活出生命Live演唱会 今天晚上的星星很少 不知道它们跑那去了 赤裸裸的天空 星星多寂廖 我以为伤心可以很少 我以为我能过的很好 谁知道一想你 思念苦无药 无处可逃 想念你的笑 想念你的外套 想念你白色袜子 和你身上的味道 我想念你的吻和手指淡淡烟草味道 记忆中曾被爱的味道
今天晚上心事很少 不知道这样算好不好 赤裸裸的寂寞 朝着心头绕 我以为伤心可以很少 我以为我能过的很好 谁知道一想你 思念苦无药 无处可逃 想念你的笑 想念你的外套 想念你白色袜子 和你身上的味道 我想念你的吻和手指淡淡烟草味道 记忆中曾被爱的味道 想念你的笑 想念你的外套 想念你白色袜子 和你身上的味道 我想念你的吻和手指淡淡烟草味道 记忆中曾被爱的味道 这首歌是张学友活出生命Live演唱会上翻唱辛晓琪的。辛晓琪这首歌是1994年出来的,在上高中时,早晨广播中有时候就会播放这首歌。听到辛晓琪的“味道”,还真没有什么味道,总觉得她的声音不对,总觉得有点像一个怨妇在回忆。如果这首歌让张惠妹,或者声音不那么尖的人唱会更好一点。而张学友版的“味道”,真的唱出了“味道”的“味道”。一种成熟的人,面对自己的过去,面对自己空荡荡的内心。这是一种理性的回味,是一种让人怜爱的心痛。能让人听出她的不舍,听出她内心的伤痛。所以,这个男版的味道才真正的唱出了这首歌的意境和胸襟。 这首歌也是年纪稍微大一点的人所喜欢的歌曲。所以,在这里解析,也算是比较有代表性,代表了70后的人对情感的回味。 其实听这首歌就可以感受到很重的70后气息。这么投入的女人,想念外套,问题是还想念袜子。我觉得我们八零,九零的,那有空去想起来谁的白色袜子啊。但是,这样的风格适合九十年代初的风格,那时候,流行音乐还是比较新鲜的东西,人们抒情也比较委婉。这样的歌曲在当时已经是很够意思了。 另外一点,我要补充的是,我保证,就在今天之前,我还不知道是谁作的词,就刚才才查的,又是姚谦。他在那个时候已经如此牛了。这首歌已经传唱
100首红歌歌词大全
红歌100首(歌词大全) 1.十送红军 (合)一送里格红军 介支个下了山 秋风里格细雨.. 介支个缠绵绵.. 山上里格野鹿 声声哀号 树树里个梧桐 叶呀叶落光 问一声亲人红军啊.. 几时里格人马 介支个再回山 (男)三送里格红军 介支个拿那山.. 山上里格包谷.. 介支个金灿灿 包谷种子 介支个红军种 包谷棒棒咱们就能掰.. 紧紧拉着红军手 红军啊 撒下的种子 介支个红了天 (女)五送里格红军 介支个过了坡 鸿雁里格阵阵 介支个空中过 鸿雁里格能够 捎书信 鸿雁里格飞到天涯海角千言万语嘱咐 红军啊 捎信里格多保.. 介支格革命说 (女)七送里格红军 介支个五斗江 江上里格船儿 介支个穿梭忙 千军万马 介支个江畔站 十万百姓泪汪汪思情似海不能忘 红军啊 革命成功 介支个早回乡 (男)九送红军上大道 锣儿无声鼓不敲000 鼓不敲 双双里格拉着长茧的手 心像里格黄莲脸在笑 血肉之情怎能忘 红军啊 盼望里个早日.. 介支个传捷报 (合)十送里格红军 介支个望月亭 望月里格边上 介支个搭高台 高台里格十丈 白玉柱 雕龙里格画凤 放呀放光彩 朝也盼来晚也想 红军啊 这台里格名叫.. 介支个望红台 2.红军战士想念毛主席 抬头望见北斗星 心中想念毛泽东 想念毛泽东 迷路时想你有方向 黑夜里想你照路程 黑夜里想你照路程 湘江岸你燃起火炬冲天 亮 号召工农闹革命 井岗山你率领我们打天 下 红旗一展满地红 抬头望见指路星 心中想念毛泽东 想念毛泽东 困难时想你有力量 胜利时想你心里明 胜利时想你心里明 瑞金城你首创革命根据 地 工农掌权好威风 赣江边你率领我们反围 剿 杀败蒋匪百万兵 啊... 红军是你亲手创 战略是你亲手定 革命战士怀念你 伟大的领袖毛泽东 革命战士怀念你 伟大的领袖毛泽东 3.红星歌 红星闪闪放光彩 红星灿灿暖胸怀 红星是咱工农的心 党的光辉照万代 红星是咱工农的心 党的光辉照万代 长夜里红星闪闪驱黑暗 寒冬里红星闪闪迎春来 斗争中红星闪闪指方向 征途上红星闪闪把路开 红星闪闪放光彩 红星灿灿暖胸怀 跟着毛主席跟着党 闪闪的红星传万代 跟着毛主席跟着党 闪闪的红星传万代 4.映山红 夜半三更哟盼天明 寒冬腊月哟盼春风 若要盼得哟红军来 岭上开遍哟映山红 若要盼得哟红军来 岭上开遍哟映山红 岭上开遍哟映山红 岭上开遍哟映山红 夜半三更哟盼天明 寒冬腊月哟盼春风 若要盼得哟红军来 岭上开遍哟映山红 若要盼得哟红军来 岭上开遍哟映山红 岭上开遍哟映山红 若要盼得哟红军来 岭上开遍哟映山红 岭上开遍哟映山红 岭上开遍哟映山红 5.情深谊长 五彩云霞空中飘 天上飞来金丝鸟 五彩云霞空中飘 天上飞来金丝鸟 诶-…诶-… 红军是咱们的亲兄弟 长征不怕路途遥 诶-…诶-… 索玛花儿一朵朵 红军从咱家乡过 红军走的是革命的路 革命的花儿开在咱心窝 五彩云霞空中飘 天上飞来金丝鸟 诶-…诶-… 红军走的是革命的路 革命的花儿开在咱心窝 1 / 18
c语言考试试题以及答案
1. 编程求和1-2+3-4+5-6+…+99-100 #include<> int main() { int i,t,s; s=0; t=1; for(i=1;i<=100;i++) { t=-t; s=s+(-t)*i; } printf("%d\n",s); system("pause"); return 0; } 2.求:1+(1+2)+(1+2+3)+…+(1+2+3+….10) 的和 #include<> int main() { int i,t,s; s=0; t=0; for(i=1;i<=10;i++) { t=t+i; s=s+t; } printf("%d\n",s); system("pause"); return 0; } 3. 求n的值,其中a是一个不为0的数字,例如2+22+222+2222+22222,其中数字a和n由键盘输入。 #include<> int main() { int a,n,i=1,sn=0,tn=0; printf("a,n:"); scanf("%d %d",&a,&n); while(i<=n) {tn=tn+a; sn=sn+tn; a=a*10; i++; } printf("%d\n",sn); system("pause"); return 0; } 4. 有一个函数如下: x (x<5) y= 2x+6 (5<=x<15) 2x-6 (x>=15) 输入x的值,计算出相应的y值。 #include<>
int main() { int x,y; printf("输入X:"); scanf("%d",&x); if(x<5) { y=x; printf("x=%3d,y=x=%d\n",x,y); } else if(x>=5&&x<15) {y=2*x+6; printf("x=%3d,y=2*x+6=%d\n",x,y); } else {y=2*x-6; printf("x=%3d,y=2*x-6=%d\n",x,y); } system("pause"); return 0; } 5. 某国的税收政策为:1000元以下免税,1000~2000元缴纳5%的税,2000~4000元上税10%,4000元以上按20%交税。试编写程序,输入一个人的收入,计算其需要上缴的税额。 #include<> int main() { float a; scanf("%f",&a); if(a<=1000) { printf("免税",a); } if(a>1000&&a<=2000) { printf("%f",a*); } if(a>2000&&a<=4000) { printf("%f",a*); } else printf("%f",a*); system("pause"); return 0; } 6. 编程分段统计学生成绩,输入为负数时结束。要求按90-100、80-89、70-79、60-69、60以下五档分别统计各分数段人数 #include<> int main() { float score; int a[6]={0,0,0,0,0,0}; char grade; int i; do{ scanf("%f",&score);
歌词
每天爱你多一些 也曾追求也曾失落 不再有梦是你为我 推开天窗打开心锁 让希望又转动 忙碌奔波偶而迷惑 为了什么是你给我 一份感动一个理由 不疲倦不脆弱 这世界的永恒不多 让我们也成为一种 情深如海不移如山 用一生爱不完 我的爱一天比一天更热烈 要给你多些再多些不停歇 让你的生命只有甜和美OH OH 遗忘该怎么流浪 我的爱一天比一天更热烈(还要坚决) 要给你多些再多些不停歇(然后再多一些) 让恋人钟爱的每一句誓言OH OH 不再难追全都实现 MUSIC ... 心中有爱人生如歌 唱着欢乐海阔天空 来去从容不惹烦忧 有了你别无求 这世界的永恒不多 让我们也成为一种 情深如海不移如山 用一生爱不完 我的爱一天比一天更热烈 要给你多些再多些不停歇 让你的生命只有甜和美OH OH 遗忘该怎么流浪 我的爱一天比一天更热烈(还要坚决) 要给你多些再多些不停歇(然后再多一些) 让恋人钟爱的每一句誓言OH OH 不再难追全都实现 我的爱一天比一天更热烈 要给你多些再多些不停歇 让你的生命只有甜和美OH OH
遗忘该怎么流浪 我的爱一天比一天更热烈(还要坚决) 要给你多些再多些不停歇(然后再多一些)让恋人钟爱的每一句誓言OH OH 不再难追全都实现 你最珍贵 明年这个时间约在这个地点 记得带着玫瑰打上领带系上思念 动情时刻最美真心的给也不累 太多的爱怕醉 没人疼爱再美的人也会憔悴 我会送你红色玫瑰(你知道我爱流泪) 你别拿一生眼泪相对 未来的日子有你才美梦才会真一点 我学着在你爱里沉醉(我不撤退) 你守护着我穿过黑夜 我愿意这条情路相守相随你最珍贵 动情时刻最美真心的给也不累 太多的爱怕醉 没人疼爱再美的人也会憔悴 我会送你红色玫瑰(你知道我爱流泪) 你别拿一生眼泪相对 未来的日子有你才美梦才会真一点 我学着在你爱里沉醉(我不撤退) 你守护着我穿过黑夜 我愿意这条情路相守相随你最珍贵 我会送你红色玫瑰(你知道我爱流泪) 你别拿一生眼泪相对 未来的日子有你才美梦才会真一点 我学着在你爱里沉醉(我不撤退) 你守护着我穿过黑夜 我愿意这条情路相守相随你最珍贵
情歌对唱歌曲大全
一首好歌不仅能够带动KTV的气氛,说不定还能撮合几对哦~~~几百首KTV里面热门的男女对唱,好听,好唱!麦霸们请收藏! 1.《你是我心中的一首歌》王力宏selina 2.《明明很爱你》品冠梁静茹——这个歌曲好听就不说了,主要是MV很有意思,泼一身水,一巴掌,蛮搞笑的,给人感觉也是超级甜蜜的~~ 3,《好心分手》王力宏卢巧音——这歌是粤语的,所以我们唱起来有些费劲,还有一个陆毅和卢巧音的版本《至少走的比你早》,貌似是这个名字,国语的,比较好唱 4《珊瑚海》周杰伦Lara——我心情不好的时候一定会去听这个歌曲 5,《独唱情歌》Tank Selina——个人比较喜欢TANK的创作风格,很不错,很好听的歌曲~~ 6,《美丽的神话》孙楠韩红——这个歌曲是我不敢听得歌曲之一,很多回忆,尤其是成龙和金喜善的版本《神话》,听了很伤感~~ 7,《只对你有感觉》Hebe 飞轮海 8,《Way Back Into Love 》Huge Grant Haley Bennett——很有feeling的一个歌曲,品冠和梁静茹有翻唱过~~梁静茹和品冠也唱过中文版《K歌情人》~~ 9,《水晶》任贤齐徐怀钰——很早的歌曲,记忆犹新的歌词“我和你的爱情,好像水晶......”是叫人感觉爱情甜蜜的歌曲~~ 点击播放 10,《梁山伯与茱丽叶》卓文萱曹格——本来是两对情侣中各自的男女主人公,被安在一个歌曲里面,歌曲很经典~~ 11,《北极雪》陈慧琳冯德伦——此歌曲还有一个新版本,是小刚和陈慧琳的《再见北极雪》 12,《选择》叶倩文林子祥——这个歌曲我印象不深,不过歌曲应该很老,很经典~~ 13,《让梦冬眠》孙楠艾雨——很考验的一个歌曲,主要是男高音太高
C语言期末考试复习题及答案
C语言期末考试复习题及答案 一、选择题:下列各题A)、B)、C)、D)四个选项中只有一个是正 确的,请将正确的选项涂写在答案纸上。答在试卷上不得分。 (1)C语言规定:在一个源程序中,main函数的位置 D 。 A)必须在最后B)必须在系统调用的库函数的后面。 C)必须在最开始。。D)可以任意 (2) C语言中的标识符只能由字母、数字和下划线三种字符组成,且第一个字符 A 。 A)必须为字母或下划线。。B)必须为下划线。 C)必须为字母D)可以是字母、数字和下划线中的任一种字符。 (3)下面四个选项中,均是正确的八进制数或十六进制数的选项是 B 。 A)-10 0x8f -011 B) 010 -0x11 0xf1 C) 0abc -017 0xc D) 0a12 -0x123 -0xa (4) C语言中int型数据在内存中占两个字节,则unsegned int取值范围是 A 。 A)0 ~ 65535 B)0 ~ 32767 C)-32767 ~ 32768 D)-32768 ~ 327687 (5) 若有定义:int a = 7; floa x = , y = ; 则表达式x + a % 3 * (int) (x + y) % 2/4 的值是 D 。 A) B) 0.00000 C) D) (6)已知ch是字符型变量,下面不正确的赋值语句是 B 。 A)ch = 5 + 9 ; B) ch= ' a + b '; C) ch = ' \ 0 '; D) ch= '7' + '6' ; (7) 设x , y和z是int型变量,且x = 3, y = 4 , z = 5 则下面表达式中值为0的
民间小调歌词
民间小调歌词 1、十二月调情(歌词) (男唱)正月里调情正月正,我看到二小妹子俊俏又年轻,皮白肉又嫩呐,我的妹子呀,玩耍没关门,乖乖,你爱坏多少人。 (女唱)二小妹子听此言慌忙开言道,叫一声情郎哥哥听奴细根苗,大街上走一趟啊我的哥哥,小郎子长的标哎呀爱坏了奴家了。 (男唱)二月里调情龙抬头,我看到二小妹子站在大门口,打你家门前过呀我的妹子啊,两眼不睬我乖乖真叫我心难过。 (女唱)二小妹子听此言慌忙开言道,叫一声情郎哥哥听奴细根苗,不是不睬你呀,我的哥哥,大街上人太多哎呀看到了笑话我。 (男唱)三月里调情桃花杏花开,我看见二小妹子站在大门外,东张又西望啊,我的妹子啊,问你看哪个乖乖你实话跟我讲。 (女唱)二小妹子听此言慌忙开言道,叫一声情郎哥哥听奴细根苗,隔壁有个王大妈我的哥哥她会拉马扯皮条,一拉就成了。 (男唱)四月里调情大麦小麦黄,我给我的二小妹子买布做
衣裳,青纱、哔叽尼啊,我的妹子啊,两样要哪样妹子你快快跟我讲。 (女唱)二小妹子听此言慌忙开言道,叫一声情郎哥哥听奴细根苗,两样我都不要我的哥哥,只要你心肠好哎呀你常常来跑跑。 (男唱)五月里调情是端阳,我看见二小妹子站在大门旁,不高又不矮啊,我的妹子啊,不瘦又不胖乖乖谁也比不上。(女唱)二小妹子听此言慌忙开言道,叫一声情郎哥哥听奴细根苗,俺人才又不标啊,我的哥哥啊,奴人丑心肠好哎呀金钱也比不了。 (男唱):六月里调情三伏天,我看见二小妹子有点心想变,隔壁有个王老板啊,我的妹子呀,常往你家跑,乖乖你想把我抛。 (女唱)二小妹子听此言慌忙开言道,叫一声情郎哥哥听奴细根苗,哪有这件事啊,我的哥哥呀,我可对天表,变心雷打天火烧。 (男唱)七月里调情秋风凉,我看到二小妹子脸色又发黄,你得的什么病呀?我的妹子,快快你跟我将乖乖拿药去熬汤。 (女唱)二小妹子听此言慌忙开言道,叫一声情郎哥哥听奴细根苗,茶饭不想吃,我的哥哥夜晚睡不着哎呀这日子真难熬。
- C语言期末考试试题及详细答案
- MOOCC语言上考试题目及答案
- C语言期末考试试题及详细答案
- 最新C语言复习题及答案
- m语言考试习题及答案
- c语言试题及答案
- C语言程序设计期末考试复习题及答案.doc
- JAVA语言程序设计期末考试试题及答案
- C语言期末考试复习题及答案
- 大学考试—高级语言程序设计——试题库及答案
- JAVA语言程序设计期末考试试题及答案
- c语言考试试题以及答案
- 工会考试练习题m
- c语言期末考试试题及答案
- 一c语言试题及答案
- C语言程序设计练习题(含程序及参考答案)
- 2020最新大学C语言考试题及答案
- JAVA语言程序设计期末考试试题及答案
- 高级语言程序设计—考试题库及答案
- c语言200道练习题及答案
