东北大学2003级《工程热力学》期末试题(2005年7月15日星期五)

东北大学2003级《工程热力学》期末试题(2005年7月15日星期五)
Questions of Semester Examination about
Engineering Thermodynamics
答题在答题纸上进行,写清题号,不必抄题。答题语言随意。
1. Write out the Following Concepts and Laws:
(1) The first law of thermodynamics; (5%) (2) Dew point; (5%)
(3) The Kelvin-Planck statement of the second law of thermodynamics; (5%) (4) The Clausius statement of the second law of thermodynamics; (5%) (5) The distinguish between ideal and real gas; (5%)
(6) The COP (coefficient of performance) of a heat pump. (5%) 2. The piston of a vertical piston-cylinder device
containing a gas has a mass of 60kg and a cross-sectional area of 0.04 m 2, as shown in Fig. 1-66. The local atmosphere pressure is 0.97bar (0.097MPa), and the gravitational acceleration is 9.81 m/s 2. (a) Determine the pressure inside the cylinder. (b) If some heat is transferred to the gas and its volume is doubled, do you expect the pressure inside the cylinder to change? (10%)
3. What is cogeneration? Please tell me about the advantages of cogeneration. (20%)
4. An ideal Diesel cycle with air as the working fluid (figure 8-24), the volume of fresh air is
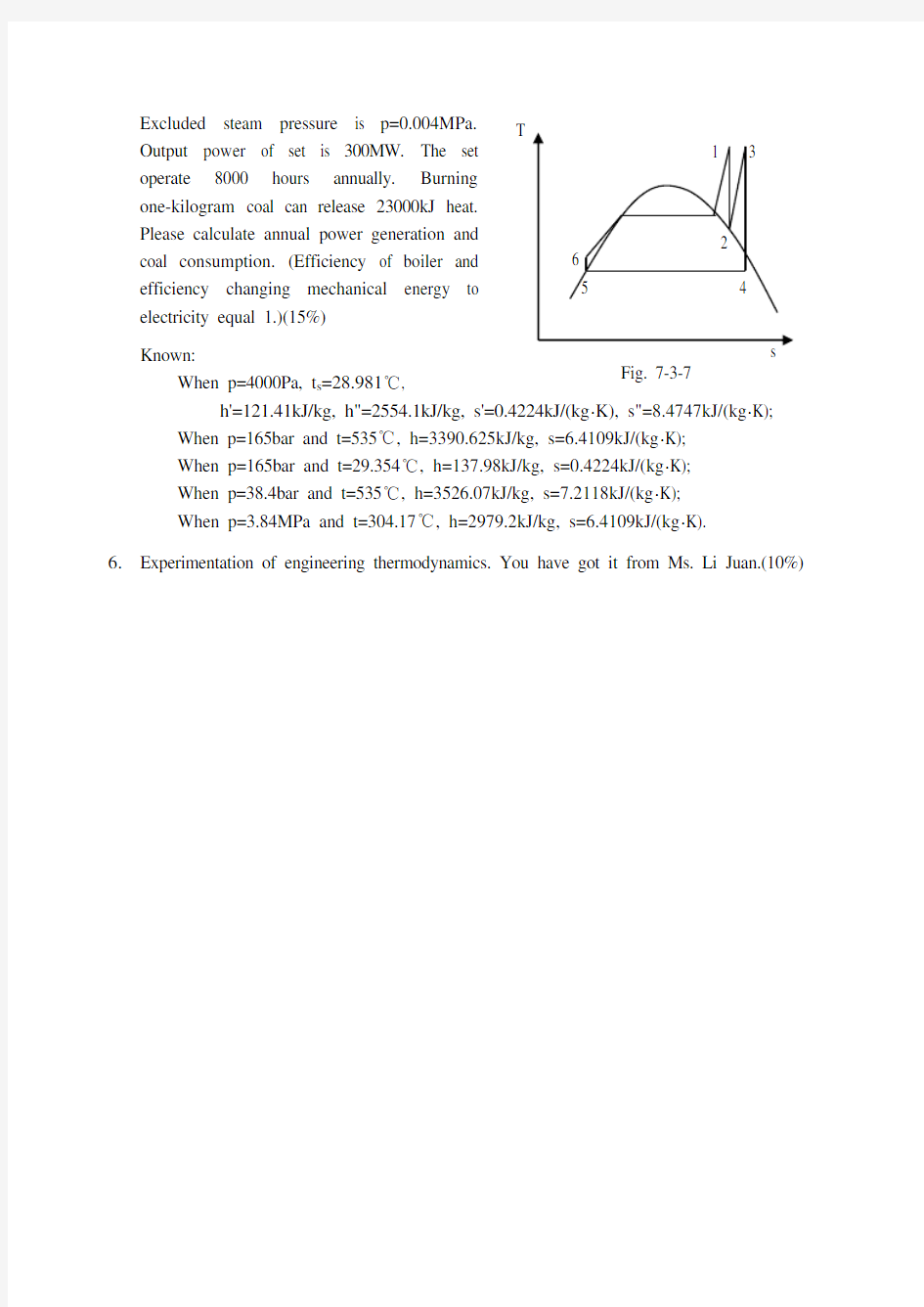
compressed to 1/18, then constant-pressure heat make volume double. At the beginning of the compression process, the working fluid is at 101325Pa, 27℃, and 1600 cm 3. Assumed the specific heats of air are constant. Determine (a) the temperature and pressure of the air at the end of each process, (b) the net work output and the thermal efficiency. (15%) 5. (Fig. 7-3-7) Fresh steam parameters of steam
power set operated with reheat cycle are p 1=16.50MPa, T 1=808.15K. Reheat pressure and temperature are p 3=3.84MPa, T 3
=808.15K.
P atm Figure 1-66 Schematic for Example 1-10, and the free-body
diagram of the piston.
Excluded steam pressure is p=0.004MPa. Output power of set is 300MW. The set operate 8000 hours annually. Burning one-kilogram coal can release 23000kJ heat. Please calculate annual power generation and coal consumption. (Efficiency of boiler and efficiency changing mechanical energy to electricity equal 1.)(15%)
Known:
When p=4000Pa, t s =28.981℃,
h'=121.41kJ/kg, h"=2554.1kJ/kg, s'=0.4224kJ/(kg ?K), s"=8.4747kJ/(kg ?K); When p=165bar and t=535℃, h=3390.625kJ/kg, s=6.4109kJ/(kg ?K); When p=165bar and t=29.354℃, h=137.98kJ/kg, s=0.4224kJ/(kg ?K); When p=38.4bar and t=535℃, h=3526.07kJ/kg, s=7.2118kJ/(kg ?K); When p=3.84MPa and t=304.17℃, h=2979.2kJ/kg, s=6.4109kJ/(kg ?K). 6.
Experimentation of engineering thermodynamics. You have got it from Ms. Li Juan.(10%)
Fig. 7-3-7
Answers
1. (1) open system; (5%)
(2) The temperature at which air becomes saturated and produces dew.
(3) The Kelvin-Planck statement of the second law of thermodynamics; (5%) (4) The Laval nozzle; (5%) (5) Ideal gas; (5%) (6)
2. A gas is contained in a vertical cylinder with a heavy piston. The pressure inside the cylinder and the effect of volume change on pressure are to be determined.
Assumptions: Friction between the piston and the cylinder is negligible.
Analysis: (a) The gas pressure in the piston-cylinder device depends on the atmospheric pressure and the weight of the piston. Drawing the free-body diagram of the piston an shown in Fig. 1-66 and balancing the vertical forces yield
pA =p atm A +W Solving for P and substituting, p =p atm +
A
mg
=0.97?105+
04
.081
.960?=111715Pa =1.12bar
(b) The volume change will have no effect on the free-body diagram draw in part (a), and therefore the pressure inside the cylinder will remain the same. 3. The mass of air in a room is to be determined.
Cogeneration plants use the same fuel source to produce both electricity and heat (steam and/or hot water). The steam and/or hot water can be used for heating or for use in a process facility adjacent to cogeneration facility, with the excess electricity sold to a local utility for it use. The utility benefits from cogeneration by gaining an additional generating source to help it meet its demand and also by purchasing the power at a rate lower than the rate at which it sells power. Whether the system is based on steam turbines, gas turbines, or a combination of both, cogeneration can achieve a significant increase in overall fuel efficiency over conventional power plants and steam generators. The advantage of cogeneration lies in the fact that both power and a transfer of heat for an intended use can be accomplished with a total fuel expenditure that is less than would be required to produce them individually. The advantages of cogeneration:
1. The big boiler has more efficiency than small one. Generally, the boiler that output power is less 5MW has efficiency 70%, and the boiler of electricity plant has 95%.
2. Energy utilized efficiency of cogeneration plants is larger than the individual sets,
so energy sources are saving.
3. 由于减少燃料消耗,从而减少污染物排放。
4. 由于大型电厂环保设施完善,所以减少污染物排放。
5. 由于大型电厂远离居住区,所以排放的污染物对人们身体健康影响小。
6. 减少小锅炉、煤贮存、灰贮存占用的土地,尤其是中心城区的土地。
7. 减少人工费用。
8. 减少居民区布置锅炉和煤、灰运输带来的安全隐患。
4. m=
001883.01
1
1=T R V p g kg p 1=101325Pa; T 1=300K; V 1=1600cm 3=0.0016m 3 p 2=p 1k
V
V ???
? ??2
1
=101325?181.4=57955596Pa; T 2= T 11
21-???
?
??k V
V =300?181.4-1=953.30K
p 3=p 2; T 3=
g
R V p 33=28790016
.0579*******??=g R V p =1906.53K p 4=p 3k
V
V ???
? ??43
=5795596?9-1.4=267398Pa; T 4= T 31
43-???
? ??k V
V =1906.53?9-(1.4-1)=791.67K
Q 23=mc p (T 3-T 2)=1.8039kJ
Q 41=mc v (T 1-T 4)=-0.6638kJ
W net =∑Q=1.8039-0.6638=1.1401kJ
ηt =8039
.11401
.123=Q W net =63.20%
5. ηt =2
361541211h h h h h h Q Q Q W
-+---=-= h 1=3390.625kJ/kg h 2=2979.2kJ/kg h 3=3526.07kJ/kg x 4=
'4
"4'
43'4
"4'
44s s s s s s s s --=
--
4224
.04747.84224
.02118.7--=
=0.8432
h 4=x 4(h 4"–h 4')+ h 4'
=0.8432(2554.1-121.41)+121.41
=2172.56kJ/kg
Fig. 7-3-7
h 5=h 4'=121.41kJ/kg h 6=137.98kJ/kg
ηt =2
.297907.352698.137625.339041
.12156.21721-+---=46.02%
Annual power generation: W total =Time ?W=8000?300?106=2.4?109kWh = 24?108kWh Annual heat consumption: Q total =W total /ηt =2.4?109/0.4602=5.215?109kWh=1.877?1013kJ Annual coal consumption: M=Q total /Q dw =1.877?1013/23000=816.28?106kg=816280Ton 6.
东北大学2003级《工程热力学》期末试题
(2005年7月15日星期五,小语种)
答题时不必抄题,要写清题号。
一. 问答题(每题6分,共60分)
1.有一个密封的圆筒形塑料瓶,内部几乎充满了水,水
面上漂浮着一个铁瓶盖,瓶盖内的气体产生的浮力刚
好克服铁瓶盖的重量,如图所示。若将塑料瓶轻轻捏
瘪,瓶盖会怎样?
2.写出热力学第二定律的克劳修斯说法和开尔文说法。
3.用公式表示过程功(膨胀功)、技术功、流动功、内能
(热力学能)和焓之间的关系。
4.湿球温度、干球温度、露点温度哪一个最高?哪一个
最低?
5.三级压缩中间冷却的中间压力p A和p B如何确定?
6.已知:水的p1=22.120MPa,T1=64
7.3K,v1=0.00317m3/kg。试求:水的范德瓦尔
常数。(提示:数学上工质的临界点是个拐点。)
7.在可逆绝热过程中,过程的膨胀功与技术功有什么关系?内能(热力学能)变化
和焓的变化又有什么关系?
8.渐缩形喷管的进口参数不变时,逐渐降低出口外的背压,试分析出口压力,出口
流速及流量的变化情况。
9.什么是理想气体?
10.解释概念:热源、工质、准静态过程。
二. 计算题(每题20分,共40分)
1.如图所示可逆的热力循环,空气在1-2的绝热压缩过程中的压缩比为20,定容过
程加热量为250kJ/kg,定压过程加热量也为
250kJ/kg,4-5过程为绝热作功过程,已知
p1=0.1MPa、t1=27℃,求其热效率,再计算同
温度范围的卡诺循环热效率,比较两者的差
别。
2.压力为1MPa、温度为200℃的水蒸气,以20m/s
的速度在一绝热喷管内作稳定流动,喷管出口
蒸气压力为0.6MPa,温度为170℃。已知:
1MPa、200℃时,h1=2827.3kJ/kg,
v1=0.2059m3/kg;0.6MPa、170℃时,h2=2782.6kJ/kg,v2=0.3257m3/kg。试求:①出口处气流速度;②当进口速度近似取作零时,出口速度为多少?百分误差多少?③进出口截面面积比A1/A2。
答案
一 问答题
1 圆筒形捏瘪后,体积缩小;水是不可压缩流体,所以气体的体积变小,瓶盖内气
体的浮力减少(排水量减少),瓶盖下沉。
2 克劳修斯说法:热不可能自发地、不付代价地、从低温物体传到高温物体。
开尔文说法:不可能制造出从单一热源吸热,使之全部转化成功而不留下其他任何变化的热力发动机。
3 w=?(pv)+w t , h=u+pv, ?h+w t =?u+w
4 干球温度>湿球温度>露点温度
5 设压缩前后压力为p 1、p 2,则p A =221p p ,p B =221p p
6 范德瓦尔方程为2v
a
b v RT p --=
,a 、b 称为范德瓦尔常数,与R 类似,决定于工质的性质。数学上,拐点有0=??? ????c T v p 和022=???? ????c
T v p ,所以 ()023
2=+--=???
????c
c c T v a b v RT v p c ()0624322=--=???? ????c c
c T v a b v RT v p c 解得:a =2
2
236427c c C
C v p p T R =
b =
C C p RT 8=3
c
v 对于水:范德瓦尔常数a =2
3c c v p =3?22.12?106?0.003172= 666.845(Pa ?m 6/kg 2)
范德瓦尔常数b =
3
00317
.03=
c v =0.001057(m 3/kg) 7 w t =kw, ?h=k ?u
8 假定进口压力为p 1,背压为p b ,当p b 从p 1向下降时,
9 所谓理想气体,是一种假想的实际上不存在的气体,其分子是一些弹性的、不占体
积的质点,分子间无相互作用力。
10 热源:工质可以从中吸取热量或向其放出热量的理想物体,其唯一特征是温度,分
为恒温热源、变温热源或高温热源和低温热源等等。 工质:在热能与机械能相互转换过程中起媒介作用的物质,要求有流动性和膨胀性。 准平衡过程(准静态过程):热力系统从一个平衡状态出发,连续经过一系列(无穷多个)平衡的中间状态,过渡到另一个平衡状态的过程。在此过程中,不存在有限势差或过程进行无限缓慢。 二 计算题
1 2
1v v
=20, v 3=v 2, q 23=250kJ/kg, p 4=p 3, q 34=250kJ/kg, p 5=p 1=0.1MPa, T 1=300.15℃
η=1-34
2351
q q q +, q 51=c p (T 1-T 5)
R=287J/(kg ?K), c p =1004J/(kg ?K), c v =717J/(kg ?K)
v 1=11p RT =6
101.015.300287??=0.86143m 3/kg, v 2=v 1/20=0.04307m 3/kg T 2=11
2
1
T v
v k -???
? ??=201.4-1?300.15=994.83K
T 3=q 23/c v +T 2=250000/717+994.83=1343.51K
p 3=33v RT =04307.051.1343287?=8.9526MPa=p 4
T 4=q 34/c p +T 3=250000/1004+1343.51=1592.51K
T 5=T 44
.114.11
45
9526.81
.051.1592--?
?
? ???=?
??
? ??k
k p p =440.94K
q 51= c p (T 1-T 5)=1.004?(300.15-440.94)=141.36kJ/kg
η=1-342351
q q q +=1-141.36/(250+250)=0.7173
ηc =1-51.159215.300141-=T T =0.8115
2 β2=
1
6
.012=
p p =0.6>βcr , 为渐缩喷管。 c 2=()()232
1
2120106.27823.282722+?-=+-c h h =299.667m/s c 2’=()()321106.27823.282722?-=-h h =298.998m/s, 相差0.669m/s, 百分误差为0.669/299.667=0.22%
因为222111v c A v c A =,所以3257.0202059.0988.298211221??==v c v c A A = 9.472
东北大学软件工程硕士《软件项目管理》复习题201407
软件工程硕士《软件项目管理》复习题201407 (答题要点,略有差异) 第一部分重要知识点 第一章项目管理概述 1、项目的属性有哪些?项目与日常工作有什么不同? 特定的目的、临时性、渐进性、需要来自不同领域的资源、有一个主要客户或发起人、不确定性 2、项目管理的“四项约束”是什么? 时间、范围、成本、质量 3、什么是项目干系人,通常包括哪些人员? 项目干系人是参与项目活动或受项目活动影响的人,包括项目发起人、项目团队、支持人员、客户、使用者、供应商、反对者等 4、项目经理的作用是什么?项目经理应具备哪些技能? 项目经理的主要职责是确保项目的全部工作在项目预算范围内按时、优质的完成,从而使客户满意。 项目经理是项目工作的领导者、计划者、组织者、控制者,管理项目的日常活动,为客户提供可交付成果。 项目管理知识体系;应用领域的知识、标准和规则;项目环境知识;通用管理知识和技能;软技能和人际关系能力。 5、项目经理的领导力为什么那么重要? 研究发现,出众的领导力是项目成功的重要因素,成功的项目经理所展示的领导才能有:远见卓识、专业技能、决策果断、擅于沟通和激励等。拥有领导能力的项目经理能够制定远期和近期项目目标,并能激励团队成员实现这些目标。优秀的项目经理知道项目的干系人更想要什么,能够很好的管理当前的项目,除了能够抓住重点外,还非常注重实效。 第二章项目管理和IT背景 1、简要描述职能型、项目型和矩阵型组织的区别,描述每一种结构对项目管理的影响? 2、什么类型的组织文化能够提升好的项目环境? 员工更认同组织、工作活动重视团队、团队凝聚力强、抗风险能力高、有合适的基于绩效的奖励、对不同意见的容忍程度高、有很强的开放性、注重人员、能够控制投入与产出的平衡。 3、为什么高层管理的参与对于项目的成功至关重要? 高层管理者是项目的重要干系人,能够帮助项目经理成功领导项目一个非常重要的因素是他们能够从高层管理者那里得到参与和支持的程度。没有高层管理者的参与,很多项目将会失败。具体原因如下:1)项目经理需要足够的资源,如果项目经理得到高层管理的参与,将获得足够的资源;2)项目经理经常需要及时得到对于特定项目需求的认可;3)项目经理必须与组织内其他部门的人进行合作,高层管理需干预以鼓励职能经
房屋建筑学期末考试试题及答案(20200814105554)
建筑工程技术(高起专)课程:房屋建筑学(高起专)总时长:180分钟 1. (多选题)屋顶排水方式分为()两大类。(本题3.0 分) A 、有组织 B 、人工式 C 、无组织 D 、自然式 标准答案:AC 2. (多选题)框架轻板建筑的外墙板按其构造形式和所用材料的不同,可分为()三类墙板。(本题 3.0 分) A 、复合 B 、剪力 C 、玻璃幕 D 、单一 标准答案:ACD 3. (多选题)建筑中墙体作用一般包括()(本题3.0 分) A 、承重作用
B 、围护作用 C、分隔作用 D、装饰作用 标准答案:ABCD 4. (多选题)实体砖墙的砌筑方式包括()(本题3.0 分) 一顺一丁 A、 多顺一丁 B、 C、梅花丁 D、十字式 标准答案:ABCD 5. (多选题)屋面防水主要做法有()。(本题3.0 分) 卷材防水 A、 刚性防水 B、 C、涂膜防水 D、直接防水 标准答案:ABC
6. (多选题)屋顶的设计要求包括()。(本题3.0 分) A 、功能要求 B 、结构要求 C 、建筑艺术要求 D 、装饰要求 标准答案:ABC 7. (多选题)预制楼板按支承条件可以分为()(本题3.0 分) A 、单向板 B 、双向板 C 、悬挑板 D 、空心板 标准答案:ABC 8. (多选题)窗框的安装方法有[?] 和[?] 两种。(本题3.0 分) A 、立口 B 、横口 C 、塞口
D 、斜口标准答案:AC 9. (多选题)人工地基加固处理方法有()。(本题3.0 分) A 、加固法 B 、压实法 C 、换土法 D 、打桩法 标准答案:BCD 10. (多选题)钢筋混凝土楼梯按施工方式不同,主要()两类。(本题3.0 分) A 、统一式 B 、现浇整体式 C 、预制装配式 标准答案:BC 11. (多选题)根据均衡中心的位置不同, 均衡可分为()两种形式。(本题 3.0 分) A 、对称的均衡 B 、一般均衡
东北大学大学物理2010
一、 填空题 1.已知某简谐运动的振动曲线如图所示, 则此简谐运动的运动方程为 _______________。 2. 一声源以20m/s 的速率向静止的观察者运动, 观察者接收到声波的频率是1063Hz,则该声源的 振动频率为 Hz .(声速为:340m/s) 3. 在驻波中,两个相邻波节之间各质点的振动相位_____ 。 4.一束光强为I 0的自然光依次通过三个偏振片P 1、P 2、P 3,其中P 1与P 3的偏振化方向相互垂直,P 2与P 3的偏振化方向之间的夹角为450,则通过三个偏振片后透射光强为_______________________。 5.一容器内储有氧气(视为理想气体),其压强为1.01×10 5 Pa ,温度为27 0C ,则氧气系统的分子数密度为__3 m - ;氧分子的平均平动动能为____J 。 6.1mol 理想气体由平衡态1(P 1,V 1,T )经一热力学过程变化到平衡态2(P 2,V 2,T ),始末状态温度相同,此过程中的系统熵变△S = S 2-S 1 = 。 7.在描述原子内电子状态的量子数l m l n ,,中,当4=l 时,n 的最小可能取值为_________。 8.在康普顿效应实验中,波长为0λ的入射光子与静止的自由电子碰撞后反向弹回,而散射光子的波长为λ,反冲电子获得的动能为 ______ 。 9.激光与普通光源所发出的光相比具有方向性好、单色性好、 和能量集中的特性。 二、 选择题(单选题,每小题2分,共10分) (将正确答案前的字母填写到右面的【 】中) 1.当质点以频率ν作简谐运动时,它的动能变化频率为 【 】 (A )2/ν (B )ν (C )ν2 2.处于平衡态的一瓶氦气和一瓶氮气(均可视为理想气体)的分子数密度相同,分子的平均平动动能也相同,则它们 【 】 (A )温度,压强均不相同 (B )温度相同但压强不同 (C )温度,压强都相同
外国建筑史试题及答案(同名11360)
外国建筑史 1.在古希腊建筑中,多立克柱式的檐部与柱子的比值:1:3;柱高与柱径比为:1:5.5—5.75;开间与柱径的比为:2:1;柱身有20个凹槽 2.古罗马继承了古希腊柱式,并有很大的发展,在与其拱券结构结合使用上分别出现了:券柱式、叠柱式、巨柱式、混合柱式等几种使用方式。 3.哥特建筑起源于罗曼建筑。法国最辉煌的哥特作品是:巴黎圣母院。 4.佛罗伦萨主教堂砌了12m 高的一段鼓座,鼓座墙厚4.9m。为了减少穹顶的侧推力,还采用了①穹顶轮廓采用矩形,大致是双圆心的、②用骨架圈结构、③穹顶做内外壳、④穹顶底部设一圈铁链加固等措施。 5.当代建筑的思潮与发展倾向有:高技派、结构主义、新现代主义等。 6.勒柯布西耶早期的著名的现代主义的住宅建筑是萨伏伊别墅,其典型的粗野主义的住宅作品是马赛公寓,属于象征主义的教堂建筑是朗香教堂。 7.金字塔是古埃及古王国时期的典型建筑。 雅典卫城是古希腊的神庙、群体布局以及雕刻艺术的最高成就的杰出代表。 9.被称为文艺复兴的第一朵报春花的佛罗伦萨主教堂穹顶是由伯鲁乃列斯基设计的。 v 10.罗马圣彼得教堂的穹顶是米开朗琪罗的作品。 11.风格派建筑师里德维德设计的乌德勒支住宅堪称画家蒙德里安绘画的立体化。 12.建筑大师格罗皮厄斯于1925年设计、位于德绍的包豪斯是现代建筑史上里程碑式的杰作。 13.芬兰建筑大师阿尔托的创作风格可以称之为建筑人情化,其作品代表着二战后建筑思潮中讲究“地方性与民族化”的倾向。 14.帕米欧肺病疗养院是由芬兰建筑师阿尔托设计的。 15.日本代代木的设计师是丹下健三;安藤忠雄最杰出的教堂作品是光之教堂和水之教堂。 16.埃及吉萨金字塔群其中以第四王朝第二代法老胡夫的金字塔为最大。 17.尖券和骨拱顶是哥特建筑形成的结构技术基础。 18.古希腊列雪格拉德音乐纪念亭是早期科林斯柱式的代表 19.纽约世贸大厦双塔的高度是411米,属于典雅主义风格。 20.后现代主义的隐喻主义、历史主义、装饰主义三个特征是由斯特恩提出的 21.西班牙著名伊斯兰宫殿阿尔罕布拉宫,主要有柘榴院和狮子院两个院落组成。 22.古希腊的多立克柱式,一般不设柱础,直接立于三级台基上,柱身上细下粗。 23.20世纪初提出“工业城市”规划方案并对后人产生影响的法国建筑师是加尼埃。 24.最先提出“形式随从功能”Form Follows Function的口号,并为“功能主义”的建筑设计思想开辟了道路的建筑师是沙利文,其属于芝加哥学派的建筑师。 25.在包豪斯学院里对创建现代设计基础教学很有贡献的人是格罗皮厄斯和密斯.凡.德.罗。 26.法国古典主义代表作是凡尔赛宫,设计人是于.阿.孟莎。 名词解释,可画简图解释 1、方尖碑:古埃及崇拜太阳神的实质柱式纪念碑。常成对置于神庙前。断面呈方形,长细比1:9-1:10,柱身向上渐缩,顶端覆以金字塔状物。通常为整块花岗石制成,碑身布满阴刻象形文字或图案,形式简洁,富于变化,是古埃及重要的建筑形象。 2、泰姬马哈尔陵:泰姬马哈尔陵被称为印度的一颗明珠,它是伊斯兰教的宗教建筑:陵墓全部用白色大理石建成,建在一个大坪台上。建筑形体四面对称,中央大穹窿是波斯的尖顶式,坪台四角有四座邦克楼 3、典雅主义(Fornalism):致力于运用传统的美学法则来使现代的材料与结构产生规整、端庄与典雅的庄严感。主要在美国。又称为新古典主义,新复古主义。讲究钢筋混凝土梁柱在形式上的精美。 4、伊势神宫: 5、罗马万神庙:(潘泰翁)是单一空间、集中式构图建筑的代表,也是古罗马穹顶技术的最高代表。其平面与剖面内径都是43.3m,底部墙厚6.2M,顶部有直径8.9m的圆洞。集古罗马穹顶与古希腊门式廊大全。门廊正面八棵科克林新柱子。 6、粗野主义:亦译“野性主义”或“朴野主义”。以著名建筑师勒?柯布西耶比较粗犷的建筑风格为代表的一种设计倾向。1、粗野主义同纯粹主义一样,以表现建筑自身为主,讲究建筑的形式美,认为美是通过调整构成建筑自身的平面、墙面、空间、车道、走廊、形体、色彩、质感和比例关系而获得;2、把表现与混凝土的性能及质感有关的沉重、毛糙、粗鲁作为建筑美的标准。粗野主义在建筑材料上保持了自然本色;3、以大刀阔斧的手法使建筑
软件工程-东北大学
软件工程 (学科代码: 0835) 一、学科简介与研究方向 东北大学软件工程学科是2011年2月国家首次批准调整建设的一级学科。东北大学于2011年8月设立软件工程一级学科博士学位授权点,是国家设立的第一批软件工程学科。东北大学软件工程学科的人才培养已经形成了较为完整成熟的本科生和硕士生培养体系,建立了国家软件人才国际培训(沈阳)基地、国家级人才培养模式创新实验区、辽宁省软件工程实验教学示范中心,质量工程建设取得一系列重大成果,成功培养了大批软件实用性人才。软件工程专业是省级示范专业,并被批准为国家级特色建设专业。本学科已培养了大批硕士研究生走上工作岗位,软件工程被评为“全国工程硕士研究生教育特色工程领域”。2012年,软件工程学科开始招收博士研究生,已形成了完善的本硕博贯通式软件工程人才培养体系。在全国第四轮学科评估中,东北大学软件工程学科排名全国并列第九。本学科学术队伍现有教授12人(其中博士生导师7人),副教授18人,以国家、区域科技需求为导向,结合学科的发展趋势和多年研究积累,已形成相互促进、彼此渗透、有一定优势和特色的学科研究方向。 (一)网构化软件工程及其演化技术体系。研究结合大数据的高速、多样、价值密度等特性,描述软件生态环境,分析大数据对软件工程的影响及收益,形成全新的以数据为驱动的,具有自主性、协同性、反应性、演化性和多态性相结合的软件工程理论。 (二)软件安全技术。针对软件理论和技术的研究与软件产业发展所面临的软件安全问题,围绕国家科技战略目标,立足创新研究,强调理论和应用相结合。从软件安全开发模型和软件开发的生命周期入手,重点研究安全软件工程的防护框架、软件安全防护理论与关键技术和可信软件的关键技术。 (三)基于混合现实的交互式软件开发技术。重点研究虚拟与真实空间位置映射技术、增强现实及交互技术、交互式医学信息可视化关键技术、云渲染关键技术及应用。 (四)软件定义互联网体系架构与关键技术。主要围绕着①可扩展、可信的软件定义互联网体系架构模型,②可行、高效、安全的软件定义互联网运行机制,③准确、有效的软件定义互联网量化模型与分析方法展开研究。 (五)复杂系统理论与应用技术。以混沌、分形、复杂网络等理论为基础和手段,将复杂系统理论成果和研究方法应用于计算机科学、软件工程等领域中,研究和解决软件工程领域的设计方法、可靠性分析、质量管理与预测及复杂网络与社交网络的建模、分析、挖掘、预测等问题。 (六)大数据计算与应用技术。研究高效的大数据获取、存储、管理、分析、理解和展示等方面的关键技术,包括数据密集型计算,高能效计算,非结构化数据存储和数据管
房屋建筑学期末考试试题及答案
建筑工程技术(高起专)课程:房屋建筑学(高起专)总时长:180分钟 1. ( 多选题 ) 屋顶排水方式分为()两大类。(本题3.0分) A、有组织 B、人工式 C、无组织 D、自然式 标准答案:AC 2. ( 多选题 ) 框架轻板建筑的外墙板按其构造形式和所用材料的不同,可分为()三类墙板。(本题 3.0分) A、复合 B、剪力 C、玻璃幕 D、单一 标准答案:ACD 3. ( 多选题 ) 建筑中墙体作用一般包括()(本题3.0分) A、承重作用
B、围护作用 C、分隔作用 D、装饰作用 标准答案:ABCD 4. ( 多选题 ) 实体砖墙的砌筑方式包括()(本题3.0分) A、一顺一丁 B、多顺一丁 C、梅花丁 D、十字式 标准答案:ABCD 5. ( 多选题 ) 屋面防水主要做法有()。(本题3.0分) A、卷材防水 B、刚性防水 C、涂膜防水 D、直接防水 标准答案:ABC
6. ( 多选题 ) 屋顶的设计要求包括()。(本题3.0分) A、功能要求 B、结构要求 C、建筑艺术要求 D、装饰要求 标准答案:ABC 7. ( 多选题 ) 预制楼板按支承条件可以分为()(本题3.0分) A、单向板 B、双向板 C、悬挑板 D、空心板 标准答案:ABC 8. ( 多选题 ) 窗框的安装方法有[?]和[?]两种。(本题3.0分) A、立口 B、横口 C、塞口
D、斜口 标准答案:AC 9. ( 多选题 ) 人工地基加固处理方法有()。(本题3.0分) A、加固法 B、压实法 C、换土法 D、打桩法 标准答案:BCD 10. ( 多选题 ) 钢筋混凝土楼梯按施工方式不同,主要()两类。(本题3.0分) A、统一式 B、现浇整体式 C、预制装配式 标准答案:BC 11. ( 多选题 ) 根据均衡中心的位置不同,均衡可分为()两种形式。(本题3.0分)
东北大学自控原理期末试题(2009A)答案
自动控制原理期末试题(A )卷答案 一.概念题(10分) (1)简述自动控制的定义。 (2)简述线性定常系统传递函数的定义。 解: (1)所谓自动控制是在没有人的直接干预下,利用物理装置对生产设备或工艺过程进行合理的控制,使被控制的物理量保持恒定,或者按照一定的规律变化。(5分) (2)零初始条件下,输出量的拉氏变换与输入量的拉氏变换之比。(5分) 二.(10分)控制系统如图1所示,其中)(s W c 为补偿校正装置,试求该系统闭环传递函数)()(s X s X r c ,并从理论上确定如何设计补偿校正装置)(s W c 可以使系统补偿后的给定误差为零。 图1 控制系统结构图 解: []) ()(1) ()()()()()(2121s W s W s W s W s W s X s X s W c r c B ++= = (5分) 由此得到给定误差的拉氏变换为 )() ()(1) ()(1)(212s X s W s W s W s W s E r c +-= 如果补偿校正装置的传递函数为 ) (1 )(2s W s W c = (5分) 即补偿环节的传递函数为控制对象的传递函数的倒数,则系统补偿后的误差 0)(=s E 三.(10分)已知某三阶单位负反馈系统具有一个有限零点为-1.5、三个极点分别为6.12.1j ±-和-1.49、且系统传递函数根的形式放大系数为4。试求系统在单位阶跃函数作用下,系统的动态性能指标超调量 %σ、调整时间s t 和峰值时间m t 。 解: 49.13-=s 与5.11-=z 构成偶极子可相消,故系统可以用主导极点2,1s 构成的低阶系统近似(1分) :
东北大学《软件工程与UML建模》期末考试必备真题集(含答案)44
软件工程与UML建模复习题A 一:单选题( 1.是在系统之外,透过系统边界与系统进行有意义交互的任何事物 A).相关系统B).Use Case C).Class D).Actor 2.软件工程是以为核心 A).过程B).面向对象C).软件开发D).质量 3.“系统应具有很高的可靠性,使用该产品的前3个月,系统不应该出现崩溃(数据不可恢复)的现象”,这属于 A).功能性需求B).客观需求C).主观需求D).非功能性需求 4.“系统每天晚上自动生成进货报表”,Actor是: A).系统B).其它系统C).时间D).报表审阅者 5.数据流程图是一个分层的概念模型,分三个层次:,分别描述系统的不同特征 A).总体图、二级图、三级图B).总体图、二级图、细节图 C).总体图、零级图、细节图D).总体图、次级图、细节图 6.以下用例命名中,最合理的是 A).进行宠物搜索B).查询宠物C).宠物查询D).进行宠物查询 7.某系统中有两个用例:一个用例的参与者是用户,用例是“注册”;另一个用例的参与者是系统管理员,用例是“审核用户注册”。这两个用例之间是什么关系? A).包含关系B).没有关系C).扩展关系D).泛化关系
8.在软件的层次结构中,“一个模块被其他模块直接调用的调用者的数量”是指 A).深度B).扇入C).扇出D).耦合 9.设C(X)定义问题X的复杂性函数,E(X)定义解决问题X所需要工作量的函数,对于两个问题p1和p2,一般情况下如果C(p1) 《房屋建筑学》试题 一、单项选择题(每小题2分,共10分) 1. 建筑是建筑物和构筑物的统称,()属于建筑物。 A. 住宅、堤坝等 B. 学校、电塔等 C. 工厂、 C. 工厂、展览馆等 2. 建筑的构成三要素中()是建筑的目的,起着主导作用。 A. 建筑功能 B. 建筑的物质技术条件 C. 建筑形象 D. 建筑的经济性 3. ()的基数为3M与6M,其相应尺寸为300mm、600mm。 A. 水平扩大模数 B. 竖向扩大模数 C. 水平基本模数 D. 竖向基本模数 4. 平面利用系数=使用面积/建筑面积×100%,其中使用面积是指除结构面积之外的() A. 所有使用房间净面积之和 B. 所有使用房间与辅助房间净面积之和 C. 所有房间面积与交通面积之和 5. 建筑立面的虚实对比,通常是由()来体现。 A. 装饰材料的粗糙与细腻 B. 建筑色彩的深浅变化 C. 形体凹凸的光影变化 D. 门窗的排列组合 6. 地下室墙面防水有()等作法。 A. 涂沥青两遍 B. 设置多道水平防水层 C. 外贴防水卷材 D. 外墙采用防水混凝土 7. 房间的剖面形状主要是根据()要求确定的。 A. 使用功能 B. 物质技术 C. 经济条件 D. 空间艺术效果 8. 空斗墙一般适用于()场合。 A. 建筑物常受振动荷载 B. 地基土质较坚硬 C. 门窗洞口尺寸较小 D. 建筑层数较多 9. 根据建筑功能要求,()的立面适合采用以虚为主的处理手法。 A. 电影院 B. 体育馆 C. 博物馆 D. 纪念馆 10. 中型以上的热加工厂房,如轧钢、铸工、锻造等,应采用()平面布置形式。 A. 矩形 B. L 形 C. 正方形 D. 山形 东北大学大学物理期末 一、 填空题 1. 已知两分振动的振动方程分别为:t x ωcos 1= 和 )2 cos( 32π ω+=t x , (其中 x 的单位为m ,t 的单位为s ),则合振动的振幅为A = ____2___m 。 2. 在驻波中,设波长为λ,则相邻波节和波腹之间的距离为_____ 4 λ ____ 。 3.火车A 行驶的速率为20m/s ,火车A 汽笛发出的声波频率为640Hz ;迎面开来另一列 行驶速率为25m/s 的火车B ,则火车B 的司机听到火车A 汽笛声的频率为 730 Hz . (空气中的声速为: 340m/s) 4.在空气中,用波长为λ= 500 nm 的单色光垂直入射一平面透射光栅上,第二级缺级 光栅常数 d =2.3×10 -3 mm ,则在观察屏上出现的全部主极大条纹条数为__5 _条。 5.光的偏振现象说明光波是____横波______。 6.一体积为V 的容器内储有氧气(视为理想气体,氧气分子视为刚性分子),其压强为P ,温度为T ,已知玻耳兹曼常数为k 、普适气体常数(摩尔气体常数)为R , 则此氧气系统的分子数密度为__ kT p ___ 、此氧气系统的内能为___pV 2 5 ____。 7.处于平衡态A 的理想气体系统,若经准静态等容过程变到平衡态B ,将从外界吸热416 J ; 若经准静态等压过程变到与平衡态B 有相同温度的平衡态C 时,将从外界吸热582 J , 则从平衡态A 变到平衡态C 的准静态等压过程中,系统对外界所作的功为 166 J 。 8.不考虑相对论效应,电子从静止开始通过电势差为U=300V 的静电场加速后, 其德布罗意波长为___0.07__nm 。 (电子静止质量:kg 101.931 -?=e m ;电子电量:C 10 6.119 -?=e ; 普朗克常量:s J 10 63.634 ??=-h ) 9.描述微观粒子运动的波函数ψ(r , t )须满足的条件是 单值 、连续、有限、归一。 1990中外建筑史 一、解释名词(15分) 1、 CIAM——现代建筑师国际会议,现代主义建筑师自发成立的以反折衷主义推广现代建筑理念的国际性民间组织。1928年成立于瑞士拉萨尔查兹,1959年解散,1933年提出雅典宪章,提出城市四大功能:居住、游憩、交通、工作。 2、哥特式——12世纪形成于法国,使用了尖券、尖拱、骨架券、飞扶壁、束柱、花窗 棂等,典型实例是巴黎圣母院。 3、风格派——1917年形成于荷兰,先出现于绘画,后影响建筑,或称新造型派,或新要素派,强调体形分解、线条、板面与色彩,强调构图韵律。 4、有机建筑——赖特把自己的建筑称做有机的建筑,有机建筑是一种由内而外的建筑,它的目标是整体性,强调局部与整体的统一,在布局、材料、造型上与环境有机结合,在结构与材料上要表现自然本色充分利用质感。 5、隐喻主义建筑——体现象征主义,强调艺术造型和象征含义的建筑,产生于50年代。 二、试绘图说明西方古典柱式的特征、比例及对后来建筑设计有何影响?(15分) 三、试简要说明什么是后现代主义?(20分) 四、试述中国古代城市的变化与特征,并举出其不同典型实例。(20分)答:中国古代城市的三个基本要素:统治机构、手工业和商业、居民区。城市形态随三者的发展不断变化,大致分为四个阶段。 第一个阶段是城市初生期,相当于原始社会晚期和夏商周三代,各种要素的分布还处于散漫而无序的状态,中间并有大片空白地段相隔,说明此时的城市还处于初始阶段。典型实例为河南偃师二里头的宫殿遗址。 第二个阶段是里坊制的确立期,相当于春秋至汉,城市总体布局还比较自由,形式较为多样,有的是大城包小城,如曲阜鲁故都及苏州吴王阖闾故城,有的是二城东西并列,如易县燕下都故城。 第三个阶段是里坊制极盛期,相当于三国至唐,三国时的曹魏都城——邺开创了一种布局规则严整,功能分区的里坊制城市格局,平面呈长方形,宫殿位于城北居中,全城作棋盘式分割,居民市场纳入这些棋盘格中组成“里”,这是前一阶段较自由的里坊制城市布局基础上进一步优化的结果,这样,不仅各种功能要素区划明确,城内交通方便,而且城市面貌也更为壮观,唐长安城堪称是这类城市的典范。 第四阶段是开放式街市期,即宋代以后的城市模式,里坊制的城市模式宣告消亡,代之而起的是开放式的城市布局,典型实例为汴梁。 五、独乐寺观音阁在建筑和结构上有何特点,请予以介绍。(10分) 东北大学继续教育学院 软件工程与UML建模X 试卷(作业考核线上2) A 卷 . D 是在系统之外,透过系统边界与系统进行有意义交互的任何事物 A).相关系统B).Use Case C).Class D).Actor 2.软件工程是以 D 为核心 A).过程B).面向对象C).软件开发D).质量 3.“系统开发过程和可交付文档将遵照ZCo-SP0STAN-95中相关规定”,这属于 D A).功能性需求B).客观需求C).主观需求D).非功能性需求 4.“系统每天晚上自动生成进货报表”,Actor是: C A).系统B).其它系统C).时间D).报表审阅者 5.数据流程图是一个分层的概念模型,分三个层次: C ,分别描述系统的不同特征 A).总体图、二级图、三级图B).总体图、二级图、细节图 C).总体图、零级图、细节图D).总体图、次级图、细节图 6.以下用例命名中,最合理的是 B A).进行宠物搜索B).查询宠物 C).宠物查询D).进行宠物查询 7.某系统中有两个用例:一个用例的参与者是用户,用例是“注册”;另一个用例的参与者是系统管理员,用例是“审核用户注册”。这两个用例之间是什么关系? B A).包含关系B).没有关系C).扩展关系D).泛化关系 8.在软件的层次结构中,“一个模块被其他模块直接调用的调用者的数量”是指 B A).深度B).扇入 C).扇出 D).耦合 9.设C(X)定义问题X的复杂性函数,E(X)定义解决问题X所需要工作量的函数,对于两个问题p1和p2,一般情况下如果C(p1) 河南工程学院 2017年秋《房屋建筑学》期末试题 批次专业:2016年春季-建筑工程技术(高起专)课程:房屋建筑学(高起专)总时长:180分钟 1. ( 多选题 ) 屋顶排水方式分为()两大类。(本题分) A、有组织 B、人工式 C、无组织 D、自然式 标准答案:AC 2. ( 多选题 ) 框架轻板建筑的外墙板按其构造形式和所用材料的不同,可分为()三类墙板。(本题分) A、复合 B、剪力 C、玻璃幕 D、单一 标准答案:ACD 3. ( 多选题 ) 建筑中墙体作用一般包括()(本题分) A、承重作用 B、围护作用 C、分隔作用 D、装饰作用 标准答案:ABCD 4. ( 多选题 ) 实体砖墙的砌筑方式包括()(本题分) A、一顺一丁 B、多顺一丁 C、梅花丁 D、十字式 标准答案:ABCD 5. ( 多选题 ) 屋面防水主要做法有()。(本题分) A、卷材防水 B、刚性防水 C、涂膜防水 D、直接防水 标准答案:ABC 6. ( 多选题 ) 屋顶的设计要求包括()。(本题分) B、结构要求 C、建筑艺术要求 D、装饰要求 标准答案:ABC 7. ( 多选题 ) 预制楼板按支承条件可以分为()(本题分) A、单向板 B、双向板 C、悬挑板 D、空心板 标准答案:ABC 8. ( 多选题 ) 窗框的安装方法有[]和[]两种。(本题分) A、立口 B、横口 C、塞口 D、斜口 标准答案:AC 9. ( 多选题 ) 人工地基加固处理方法有()。(本题分) B、压实法 C、换土法 D、打桩法 标准答案:BCD 10. ( 多选题 ) 钢筋混凝土楼梯按施工方式不同,主要()两类。(本题分) A、统一式 B、现浇整体式 C、预制装配式 标准答案:BC 11. ( 多选题 ) 根据均衡中心的位置不同,均衡可分为()两种 形式。(本题分) A、对称的均衡 B、一般均衡 C、不对称的均衡 标准答案:AC 12. ( 多选题 ) 窗框的安装方法有[]和[]两种。(本题分) A、立口 实验一霍尔效应及其应用 【预习思考题】 1.列出计算霍尔系数、载流子浓度n、电导率σ及迁移率μ的计算公式,并注明单位。 霍尔系数,载流子浓度,电导率,迁移率。 2.如已知霍尔样品的工作电流及磁感应强度B的方向,如何判断样品的导电类型? 以根据右手螺旋定则,从工作电流旋到磁感应强度B确定的方向为正向,若测得的霍尔电压为正,则样品为P型,反之则为N型。 3.本实验为什么要用3个换向开关? 为了在测量时消除一些霍尔效应的副效应的影响,需要在测量时改变工作电流及磁感应强度B的方向,因此就需要2个换向开关;除了测量霍尔电压,还要测量A、C间的电位差,这是两个不同的测量位置,又需要1个换向开关。总之,一共需要3个换向开关。 【分析讨论题】 1.若磁感应强度B和霍尔器件平面不完全正交,按式(5.2-5)测出的霍尔系数比实际值大还是小?要准确测定值应怎样进行? 若磁感应强度B和霍尔器件平面不完全正交,则测出的霍尔系数比实际值偏小。要想准确测定,就需要保证磁感应强度B和霍尔器件平面完全正交,或者设法测量出磁感应强度B 和霍尔器件平面的夹角。 2.若已知霍尔器件的性能参数,采用霍尔效应法测量一个未知磁场时,测量误差有哪些来源? 误差来源有:测量工作电流的电流表的测量误差,测量霍尔器件厚度d的长度测量仪器的测量误差,测量霍尔电压的电压表的测量误差,磁场方向与霍尔器件平面的夹角影响等。实验二声速的测量 【预习思考题】 1. 如何调节和判断测量系统是否处于共振状态?为什么要在系统处于共振的条件下进行声速测定? 答:缓慢调节声速测试仪信号源面板上的“信号频率”旋钮,使交流毫伏表指针指示达到最大(或晶体管电压表的示值达到最大),此时系统处于共振状态,显示共振发生的信号指示灯亮,信号源面板上频率显示窗口显示共振频率。在进行声速测定时需要测定驻波波节的位置,当发射换能器S1处于共振状态时,发射的超声波能量最大。若在这样一个最佳状态移动S1至每一个波节处,媒质压缩形变最大,则产生的声压最大,接收换能器S2接收到的声压为最大,转变成电信号,晶体管电压表会显示出最大值。由数显表头读出每一个电压最大值时的位置,即对应的波节位置。因此在系统处于共振的条件下进行声速测定,可以容易和准确地测定波节的位置,提高测量的准确度。 2. 压电陶瓷超声换能器是怎样实现机械信号和电信号之间的相互转换的? 答:压电陶瓷超声换能器的重要组成部分是压电陶瓷环。压电陶瓷环由多晶结构的压电材料制成。这种材料在受到机械应力,发生机械形变时,会发生极化,同时在极化方向产生电场,这种特性称为压电效应。反之,如果在压电材料上加交变电场,材料会发生机械形变,这被称为逆压电效应。声速测量仪中换能器S1作为声波的发射器是利用了压电材料的逆压电效应,压电陶瓷环片在交变电压作用下,发生纵向机械振动,在空气中激发超声波,把电信号转变成了声信号。换能器S2作为声波的接收器是利用了压电材料的压电效应,空气的振动使压电陶瓷环片发生机械形变,从而产生电场,把声信号转变成了电信号。 东南历史真题 2008年 1。北京故宫中轴线建筑及院落空间特点,及其建筑功能,画出太和殿立面。20分 2.明北京,明南京城市特点及城市形成过程图20分 3.中国塔的类型及画图15分 4.中国50年代和80年代建筑发展特点20 5.画图帆拱巴黎圣母院郞香教堂透视卫城总平面罗马五柱式柱头15 6.密斯的建筑思想及作品20 7圣马可广场特点,图每个建筑形式特点20 8.当代西方建筑思潮及其发展趋势20 2007年 1。明南京特点(附图)20分 2。留园特点与成就(附图)20分 3。清七架椽举架特点(附图)10分 4。北京四合院特点(附图)10分 5。名词解释:李诫、宇文恺、戈裕良、计成、吕彦直。15分 6。雅典卫城及帕提农成就特点(附图)20分 7。巴罗克和洛可可特点比较。10分 8。当代建筑思潮和趋势。20分 9。名词解释:斯卡摩齐、维特鲁威、维尼奥拉、勒诺特、赫尔佐格。10分 10。绘图:帆拱、圣马可广场、巴黎圣母院立面、帕拉第奥母题、泰姬陵立面。15分 2006年 一、画图(25分) 1、中国古代五种屋顶的形式(悬山、歇山、硬山、庑殿、攒尖) 2、天坛的总平面 2、山屋角剖面和仰平面 4、北京四合院 5、中山陵总平面 二、画图(25分) 1、帆拱透视 2、泰姬陵透视 3、圣马可广场 4、巴黎圣母院立面 5、罗马五柱式 三、雅典卫城的布局特点、经验手法及艺术成就(附加图) 25分 四、结合拙政园谈谈南方园林造园手法 25分 五、故宫的艺术成就和单体的成就(附加图) 25分 六、谈谈西方近现代建筑思潮 25分 2005年 一:雅典卫城和帕提农神庙; 二:天坛的特点; 三: 1.巴黎圣母院 2.拙政园 3.泰姬陵 4.五采斗拱 5.故宫太和殿 6.帆拱四:圣马可广场; 五:中山陵; 六:花园城市理论; 2004年 雅典卫城巴黎圣母院佛光寺大殿包豪斯帆拱举折屋面画法叉柱造天坛五色图圣马可广场里坊制谈谈西方近现代建筑思潮谈谈设计结合自然并举例说明 《房屋建筑学》试题 一、选择题(每小题1分,共30分) 1.采用防水砂浆做建筑物水平防潮层不适于。 A.地基潮气大的建筑 B.地基会产生变形的建筑 C.地基埋深过大的建筑 D.地基埋深小的建筑 2.屋面泛水是指屋顶上所有防水层的铺设沿突出屋面部分的。A.沿口 B.女儿墙 C.变形缝 D.垂直面 3.常用的人工加固地基的方法不包括下列哪一项? A.压实法 B.换土法 C.桩基 D.筏形基础 4.大厅式组合一般适用于建筑类型。 A.医院、办公楼、中小学 B.剧院、电影院、体育馆 C.火车站、浴室、百货商店 D.医院、展览馆、图书馆 5.当门窗洞口上部有集中荷载作用时,其过梁可选用。 A.平拱砖过梁 B.弧拱砖过梁 C.钢筋砖过梁 D.钢筋混凝土过梁6.倒铺屋面,其保温材料为。 A.珍珠岩 B.蛭石 C.膨胀珍珠岩 D.聚氨脂发泡材料7.低层、多层住宅阳台栏杆净高不应低于 mm. A.900 B.1000 C.1050 D.1100 8.对有保温要求的墙体,需提高其构件的。 A.热阻 B.厚度 C.密实性 D.材料导热系数 9.对于大型的比较复杂的工程,建筑设计一般采用设计阶段。 A.1个 B.2个 C.3个 D.4个 10.房间的剖面形状主要是根据要求确定的。 A.使用功能 B.物质技术 C.经济条件 D.空间艺术效果 11.刚性屋面的浮筑层可用的材料为。 A.水泥砂浆 B.石灰砂浆 C.细石混凝土 D.防水砂浆 12.钢筋混凝土构造柱的作用是。 A.使墙角挺直 B.加快施工速度 C.增强建筑物的刚度 D.可按框架结构考虑 13.根据基础埋置深度的不同,基础有深基础、浅基础和不埋基础之分。当基础埋置深度大于多少时称为深基础。 A.0.5m B.2.0m C.4.0m D.5.0m 14.管线穿越楼板时,何种管线需加套管。 A.下水管 B.自来水管 C.电讯管 D.暖气管 15.基本模数的数值为。 A.600mm B.300mm C.100mm D.50mm 16.建筑的构成三要素中是建筑的目的,起着主导作用。 A.建筑功能 B.建筑的物质技术条件 C.建筑形象 D.建筑的经济性17.建筑立面的重点处理常采用手法。 A.对比 B.均衡 C.统一 D.韵律 18.建筑是建筑物和构筑物的统称,属于建筑物。 A.住宅、堤坝等 B.学校、电塔等 C.工厂、水塔 D.工厂、展览馆等 一、教材:选择填空题 1~5;计算题:13,14,18 二、附加题 (一)、选择题 1、一沿x 轴作简谐振动的弹簧振子,振幅为A ,周期为T ,振动方程用余弦函数表示, 如果该振子的初相为π3 4 ,则t =0时,质点的位置在: (A )过A x 21=处,向负方向运动; (B) 过A x 21 =处,向正方向运动; (C) 过A x 21- =处,向负方向运动; (D) 过A x 2 1 -=处,向正方向运动。 2、一物体作简谐振动,振动方程为:x =A cos(ωt +π/4 ) 在t=T/4(T 为周期)时刻,物体的加速度为: (A) 222ωA -. (B) 222ωA . (C) 232ωA -. (D) 232ωA . (二)、计算题 1、一物体沿x 轴做简谐运动,振幅A = 0.12m ,周期T = 2s .当t = 0时, 物体的位移x 0= 0.06m ,且向x 轴正向运动.求: (1)此简谐运动的运动方程; (2)t = T /4时物体的位置、速度和加速度; 2、一物体沿x 轴做简谐运动,振幅A = 10.0cm ,周期T = 2.0s .当t = 0时, 物体的位移x 0= -5cm ,且向x 轴负方向运动.求: (1)简谐运动方程; (2)t = 0.5s 时,物体的位移; (3)何时物体第一次运动到x = 5cm 处? (4)再经过多少时间物体第二次运动到x = 5cm 处? 3、若简谐振动方程为m ]4/20cos[1.0ππ+=t x ,求: (1)振幅、频率、角频率、周期和初相; (2)t =2s 时的位移、速度和加速度. 4、一简谐振动的振动曲线如图所示,求振动方程. 5、一物体沿x 轴作简谐振动,振幅为0.06m ,周期为2.0s ,当t =0时位移为0.03m ,且向轴正方向运动,求: (1)t =0.5s 时,物体的位移、速度和加速度; (2)物体从m 03.0-x =处向x 轴负方向运动开始,到达平衡位置,至少需要多少时间? 题图4 聊聊那些年研究生考试的事情。 说到关于政治,我看政治看得比较晚,八月才开始看而且看得很慢,因为我觉得看太早了也会忘记,事实证明,知识点精讲精练那本书在后期我已经忘光了……不过理科生的话建议早一点看,文科生可以九月份开始看。看一章就做一章配套练习巩固。看完精讲精练刷刷真题,就可以开始政治第二轮复习了。 这里推荐一下李凡老师的《政治新时器》教材,我只听了他的政治分析题的课,但是感觉非常有用,同时背的是她出的一本书,后半部分是各部分分析题易考考点,直接背就行。前半部分是选择题考点,后半部分是分析题,他押题押的也很准我都会,得意!最后如果有时间的话可以多做一些《政治新时器》教材押题卷,很有帮助。 英语: 我最满意的是英语,考完对答案我客观题就完形填空错了两个扣1分,别的全对,翻译和大小作文也写得很满意,小作文背到过一篇类似的。英语也是贯穿考研复习始终的,三月份开始每天早上背一小时单词和看一小时视频,单词最最最重要!背单词要坚持到考前最后一天,看视频也是为了记得更牢固,并且记更多的词组搭配。单词书我用的《一本单词》。大概到七月中旬开始做英语真题,我买的是《木糖英语真题手译》,做的时候客观题都当做考试一样做,每一年做完之后都把其中的生词记下来然后背,做完一遍之后开始第二遍,第二遍的重点主要是分析长难句以及检验单词有没有掌握牢固,蛋核英语公众号的课程也总能给我答疑。总而言之,英语关键是词汇量,每天都要坚持背单词,做起题来真的真的很不一样,轻松很多。 专业课: 首先大致浏览一遍课本,自己做一个大纲出来,大标题小标题小小标题这样,第一遍可以不细看,但要有个印象,知道书大概讲的是什么,分几章,每章又在说什么,建立一个知识框架出来。 第二遍开始仔仔细细地看课本,把这个知识框架逐步逐步地填满,不能有知识上的盲点,如果看书看不明白,就去问在校授课的专业课老师,老师会慈爱地给你详细解答。可以自己根据书本内容做笔记,做完笔记印象更深刻。 第三遍再返回去扫一下课本,可以结合真题去针对性地复习,真题的答案试 房屋建筑学 一、填空 1.构成建筑的基本要素是建筑功能、建筑技术、建筑形象。 2、屋顶排水方式可分为有组织排水、无组织排水_两大类,屋顶坡度的形成方法有材料找坡和结构找坡,坡屋顶的承重结构形式有横墙承重、屋架承重和梁架承重三种。 3.地下室砖墙须做防潮处理,墙体必须采用_水泥_砂浆砌筑,灰缝应饱满,墙身外侧设垂直防潮层。 4.伸缩缝要求将建筑物从基础以上的建筑构分开;沉降缝要求从基础到屋顶分开。当伸缩缝同时兼作防震缝时,缝宽按防震缝处理。 5.基础埋深的最小深度为_500mm_,施工规范规定的砖墙灰缝宽度为_10mm_. 6.设计程序中的两阶段设计是指_初步设计_和_施工图设计_,对于复杂工程需要增加_技术设计_阶段。 7.预制踏步根据梯段构造和支承方式的不同,可分为一字形、L形、_三角形。 8.钢筋混凝土构造柱主要从竖向加强层与层之间垂直的连接,和圈梁共同形成空间骨架。 9.为了防止因温度变化产生的裂缝无规律地开展,通常刚性防水层应设置分仓缝,其位置一般设在支座处。 10.单层厂房横向伸缩缝处应采用双柱双轴线的定位轴线划分方法,双轴线间的插入距等于变形缝宽。单层厂房的山墙为非承重墙,山墙内缘与横向定位轴线重合。 二、名词解释 1.建筑模数----- 答:选定的标准尺度单位,作为建筑物、建筑构配件、建筑制品以及有关设备尺寸相互间协调的基础。 2.刚性基础 答:由刚性材料制作的基础称刚性基础。所谓刚性材料,一般是指搞压强度高,而抗拉、抗剪强度低的材料。如砖、石、混凝土等。 3.过梁 答:当墙体上开设门、窗等洞孔时,为了支承洞孔上部砌体所传来的各种荷载,并将这些荷载传给窗间墙,常在门、窗洞孔上设置横梁,该梁称过梁。 4.泛水 答:水平屋面与垂直墙面的交接的防水处理。 5.耐火极限 答:对任一建筑构件,按照时间—温度标准曲线进行耐火试验,从受火作用时起,到构件失去稳定性或完整性或绝热性时止,这段抵抗火的作用时间,称为耐火极限,通常用小时(h)来表示。 三、简答题 1.在砖墙中,为什么要做墙身防潮层?说明细石混凝土防潮层的做法。在图1中表示外墙墙身水平防潮层和垂直防潮层的位置。 答:在砖墙中做墙身防潮层是为隔绝地下潮气等对墙身的影响。 细石混凝土防潮层,采用60mm厚的细石混凝土带,内配三根p6钢筋,其防潮性能好。(整理)《房屋建筑学》试题.
2011东北大学大学物理期末考题及答案
2019-2019东南大学建筑学考研试题共10页word资料
东北大学考试《软件工程与UML建模X》考核作业参考395
房屋建筑学期末考试答案(2016春季)
【免费下载】东北大学物理实验报告
(整理)东南大学考研建筑史真题89-08无答案.
《房屋建筑学》试题及答案
东北大学物理期末复习资料
2021东北大学软件工程考研真题经验参考书
房屋建筑学期末试题及答案
- 东北大学《公共经济学》期末考试必备真题集(含答案)46
- 东北大学期末考试题库
- 东北大学《大学语文》期末考试必备真题集(含答案)65
- 【大学期末考试复习题】东北大学马原期末试题及答案(精)
- 东北大学《大学语文》期末考试必备真题集(含答案)52
- 东北大学汇编期末试题
- 东北大学《教育管理学》期末考试必备真题集(含答案)99
- 东北大学《经济学》期末考试必备真题集(含答案)74
- 东北大学《管理学原理》期末考试必备真题集(含答案)52
- 东北大学《经济学》期末考试必备真题集(含答案) 61
- 东北大学《市政学》期末考试必备真题集(含答案)51
- 20092011下册东北大学高数期末考试试题
- 东北大学《数字电子技术基础》期末考试必备真题集(含答案)81
- 东北大学《公共组织行为学》期末考试必备真题集(含答案)80
- 东北大学《大学语文》期末考试必备真题集(含答案)28
- 东北大学期末考试试卷封皮
- 下册东北大学高数期末考试试题
- 东北大学《企业管理》期末考试必备真题集(含答案) 43
- 东北大学 东 北 大 学 期 末 考 试 试 卷( 卷)
